INTRODUCTION
CLINICAL FEATURES
PATHOGENESIS
Table 1
RADIOLOGIC FEATURES
Table 2
Simple radiography
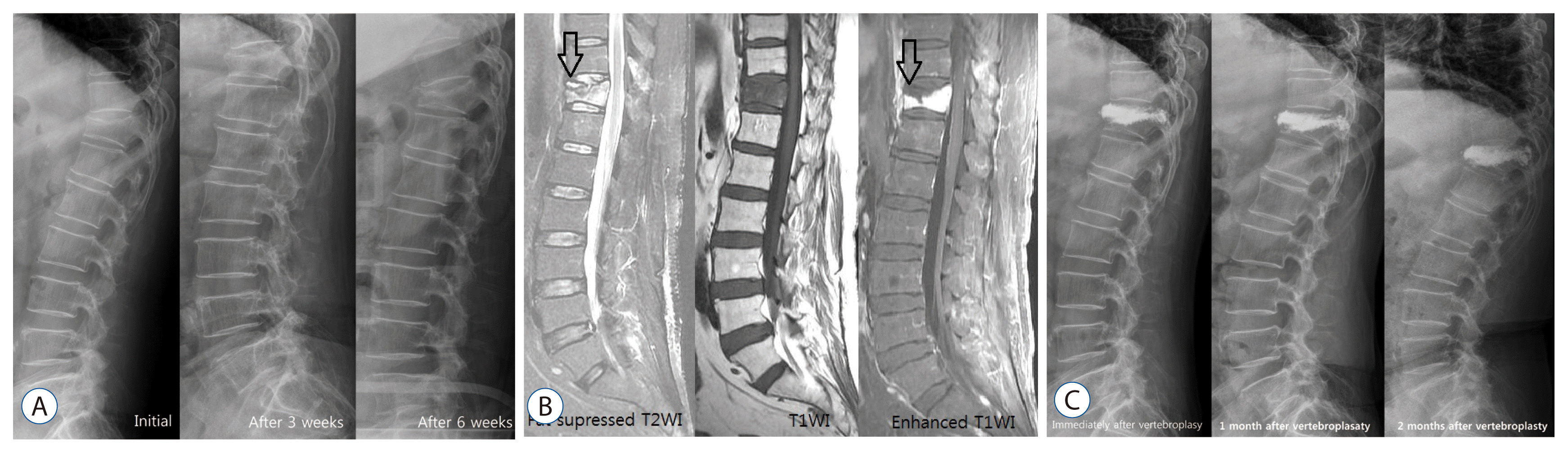 | Fig. 1Case 1. A 62-year-old woman was admitted with severe backache who was suffering from rheumatoid arthritis relying on prednisolone for many years. She was diagnosed with compression fracture of T12. Her had appearance resembling that of the Cushing’s, bone mineral density examination showed severe osteoporosis, T score of -3.7. She was followed for 3 weeks with conservative treatment and osteoanabolic agent. However, T12 vertebral compression aggravated in follow up X-ray images and vertebroplasty was performed. However simple radiography, 1 month after the vertebroplasty, showed gradual progression of compression fracture and kyphosis of the T12 vertebrae but the clinical symptoms subsided, so progressive kyphosis was followed up without additional treatment. A : Serial simple spine lateral radiography showed compression fracture of T12 and progressive kyphosis. B : MRI showed high SI in fat suppressed T2WI and diffuse low SI in T1WI of T12. As well as, MRI revealed that the fluid content of the superior endplate of T12 high SI in T2WI and the low SI area of necrotic content (black arrows : IVC sign) and enhancing vertebral body in T1WI enhanced image. C : Postoperative serial simple radiographys showed progressive kyphosis. MRI : magnetic resonance image, IVC : intraverteral cleft. |
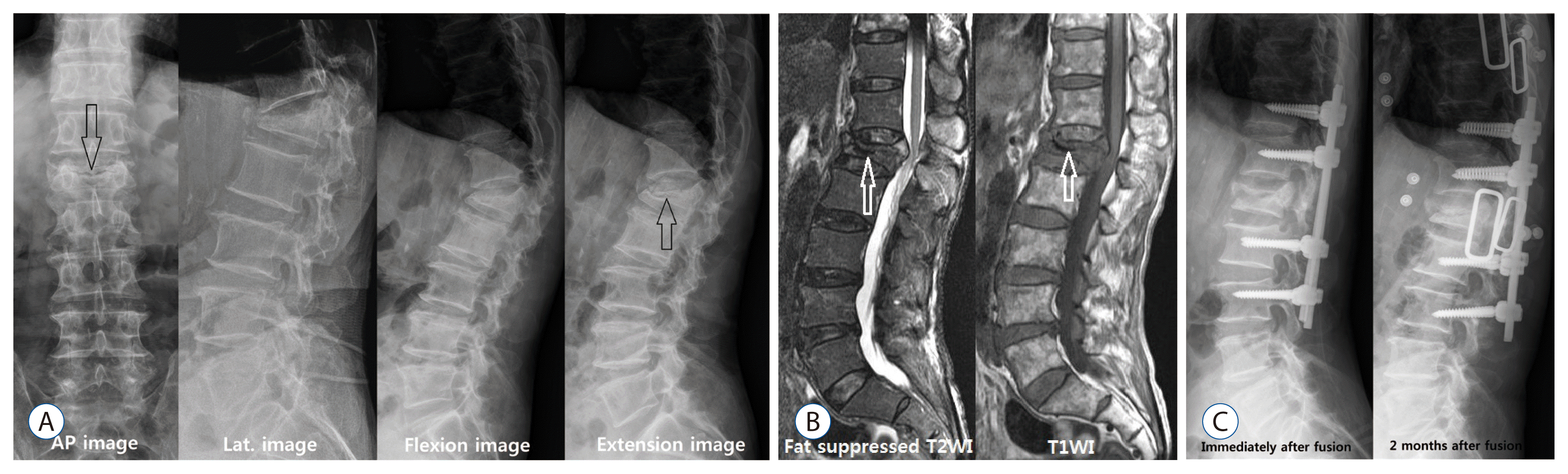 | Fig. 2A 77-year-old man presented with persistent lumbago, progressive kyphosis and radiating pain on both lower extremities lasting about 3 months after minor trauma. He underwent conservative treatment with diagnosis of L1 compression fracture 2 months ago. A : A transverse radiolucent line of IVC sign (black arrow) was observed in anteroposterior view. And the intravertebral pseudoarthrosis and IVC sign (black arrows) were observed in the flexion-extension view. B : Spine MRI showed that typical IVC sign (white arrows) and ventral portion of spinal cord was compressed due to kyphosis. At first, vertebral body replacement was considered, but long level posterolateral fusion was performed due to elder and dementia. C : The restoration of the L1 body was observed in the postoperative image. However, recollapse of the restored L1 body, L3 screw loosening and posterior pull back were observed in the image 1 month after the fusion. AP : anteroposterior view, Lat. : lateral view, IVC : intraverteral cleft, MRI : magnetic resonance image. |
Computerized tomography (CT)
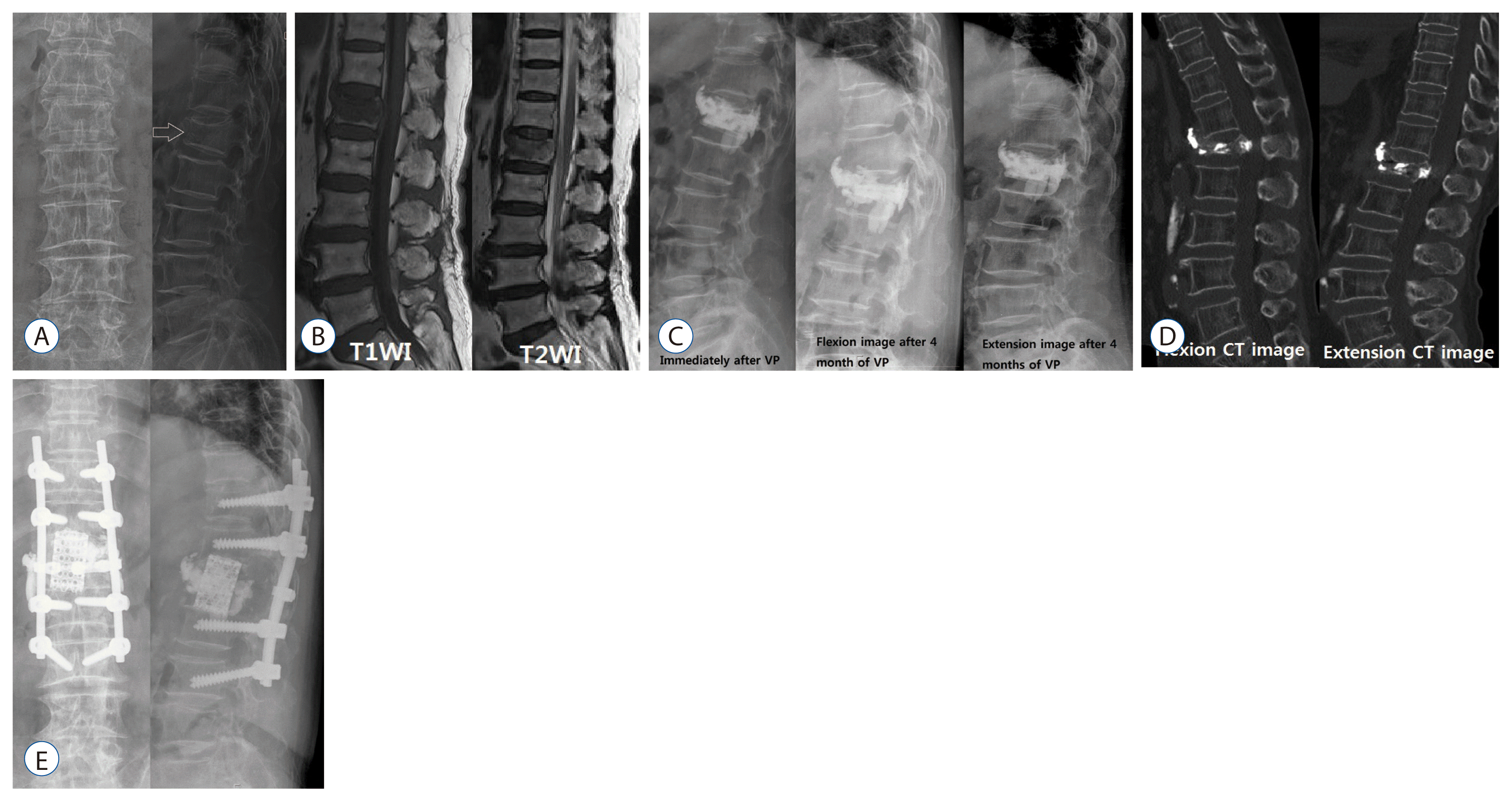 | Fig. 3A 74-year-old woman presented with persistent back pain that had fallen 2 weeks ago. Radiologic examination at the time of admission revealed recent first lumbar compression fracture (A and B) and the patient underwent vertebroplasty (C). Her symptoms were improved and she was discharged with orthrosis and biphosphonate medication. Four months later, she revisited with progressive kyphosis, persistant backache and both lower leg weaknesses. Intravertebral instability at L1 body was suspected on the thoracolumbar flexion/extension simple radiographys (C). Dynamic spine CT showed intravertebral instability at L1 and compressed thecal sac due to fractured bony fragement of L1 (D). In MRI, there was no accurate intravertebral vaccum cleft, and compressed thecal sac was observed. We thought that her pain was caused by intravertebral instability, progressive neurologic deficit was caused by more protrusion of fractured bony fragment at flexion or weight bearing. We performed L1 corpectomy with mesh cage insertion and posterolateral fusion via posterior approach (E). Neurologic deficit and persistent backache disappeared. VP : vertebroplasty, CT : computerized tomography, MRI : magnetic resonance image. |




 Citation
Citation Print
Print


 XML Download
XML Download