INTRODUCTION
Cranioplasty is usually performed for brain protection and cosmetic problem following decompressive craniectomy. Furthermore, previous studies have reported that cranioplasty improves neurological function11,12). In recent years, synthetic materials have been developed and occasionally used to replace autologous bone. However, autologous bone is still the preferred material because it has many advantages including low cost, lack of immunological response, and anatomical fit.
Cranioplasty is a relatively straightforward procedure. However, complications related to procedure including infection, wound dehiscence, postoperative hematoma or fluid collection, implant dislodgement, and bone flap resorption (BFR) have been reported in many studies1,3,4,9,13,21,22). In particular, BFR remains a long-term concern after autologous bone cranioplasty and is considered a crucial factor for reoperation. Within the literature, the rate of BFR is reported to vary greatly from 2.7% to 51%3,6,7,12,18,21). To date, only few studies have attempted to identify the risk factors related to BFR2,3,7,18).
The aim of this study was to investigate the degree and related factors of BFR following autologous bone cranioplasty.
Go to : 
MATERIALS AND METHODS
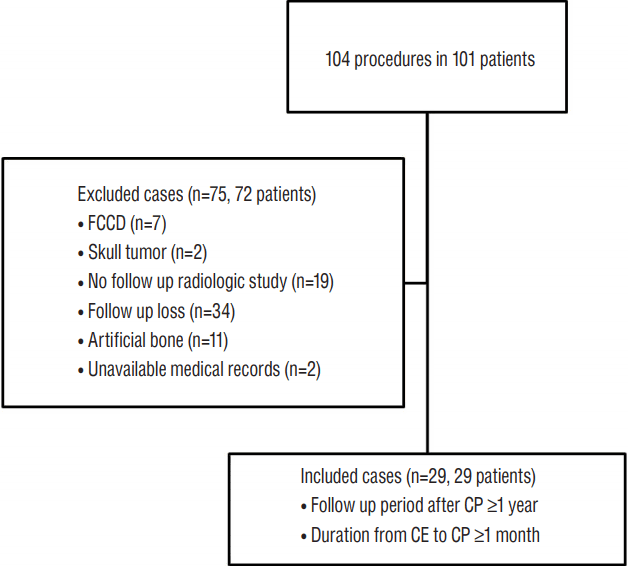
This study was designed as a single center retrospective cohort study. From April 2005 to October 2014, 101 patients who underwent cranioplasty following decompressive craniotomy for traumatic brain injury, subarachnoid hemorrhage, intracranial hemorrhage, brain tumor, and ischemic stroke were included. After excluding patients for exclusion criteria including follow-up loss, use of artificial bone, comminuted skull fracture, and skull tumor, 29 patients were included in the study (Fig. 1). Medical records were reviewed to determine initial diagnosis of craniectomy, age at cranioplasty, sex, past medical history, linear skull fracture, shunt-dependent hydrocephalus, duration from craniectomy to cranioplasty, operative time, infection, incidence of postoperative hematoma, and follow-up duration after cranioplasty.
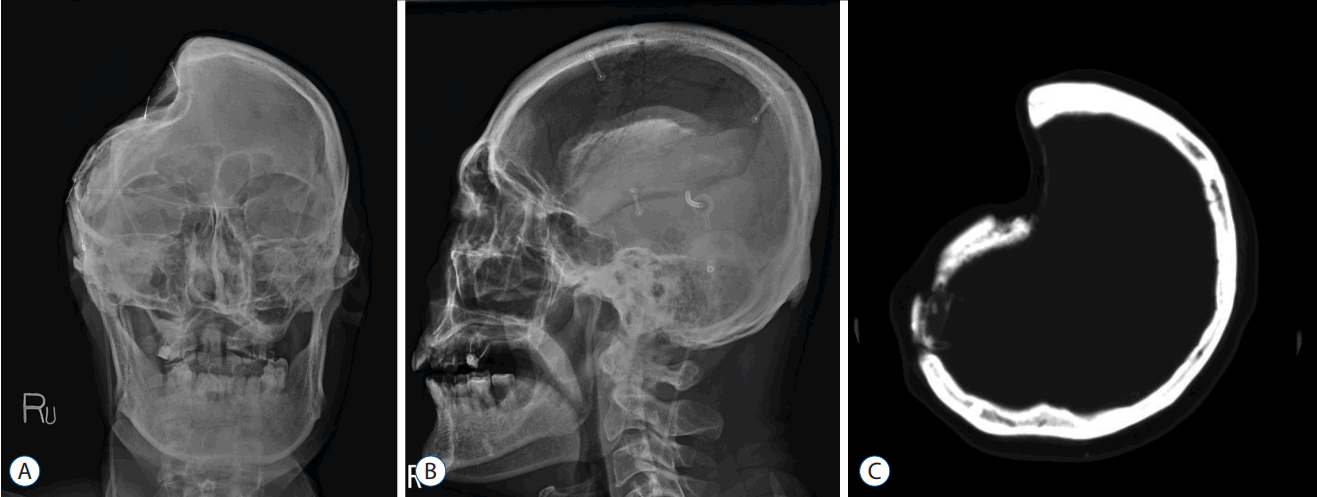
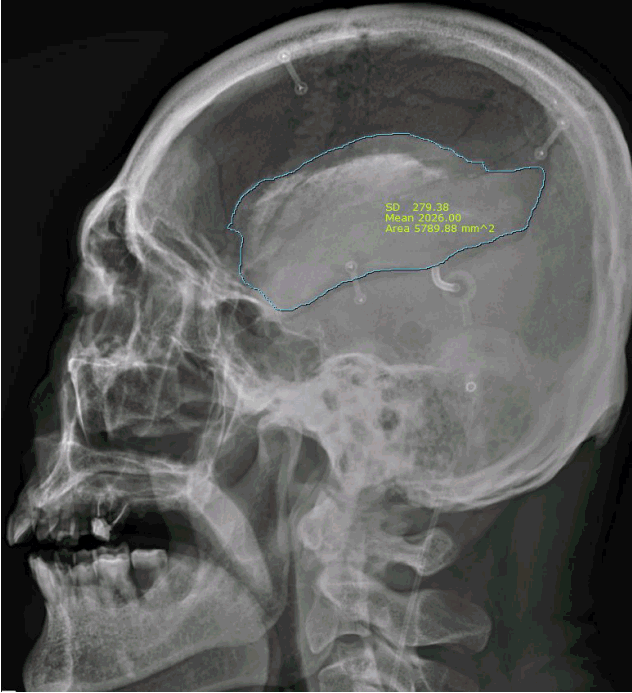
BFR was defined as the followings: 1) decrement ratio ([the ratio of initial BF/craniectomy size]–[ratio of last BF/craniectomy size]) >0.1; and 2) bone flap thinning or geometrical irregularity of bone flap shape on computed tomographic scan or skull plain X-ray (Fig. 2). The two-dimensional area was calculated on the computed tomographic scan or plain skull X-ray using the region of interest (ROI) parameter (free draw) in accordance to our institution’s order communication system program, Maroview (version 5.409; INFINITT, Seoul, Korea) (Fig. 3). The minimal interval between craniectomy and cranioplasty was one month and the minimal follow-up period was one year. BFR was determined based on the last follow up image.
We compared demographic and radiological factors between BFR and no-BFR groups. All continuous variables were presented as means±standard deviation. The Kruskal-Wallis test was used for continuous variables and Fisher’s exact test for categorical variables. Results with two-tailed values less than p=0.05 were considered statistically significant. All statistical analyses were performed using SPSS ver. 20.0 for Windows (IBM Corp., Armonk, NY, USA).
Go to : 
RESULTS
The mean age of the 29 patients was 48.1±19.7 years (male: female ratio 20: 9). Interval time between craniectomy and cranioplasty was 175.7±258.2 days and the mean follow-up period was 1364±886.8 days. A summary of the study population is described in Table 1.
Table 1
Summary of study population
BFR occurred in 8 patients (27.6%). There were no significant differences in the presence of skull fracture and shunt-dependent hydrocephalus between the BFR and no-BFR groups (Table 2). Furthermore, there were no significant differences in operative time and duration of bone flap storage between the two groups (Table 3). The overall rate of BFR was 0.10±0.11 over 3.7 years and the degree of BFR was 2.7%/year. In other words, when BFR occurs, bone flap can be estimated to be resorbed at about 2.7% per year. Following univariate analysis, younger age (30.5±23.2 vs. 54.9±13.4) and longer follow-up period (2204.5±897.3 vs. 1044.1±655.1) were significantly associated with BFR (p=0.008 and 0.003, respectively) (Fig. 4). In one patient, removal of the bone flap was carried out due to severe BFR.
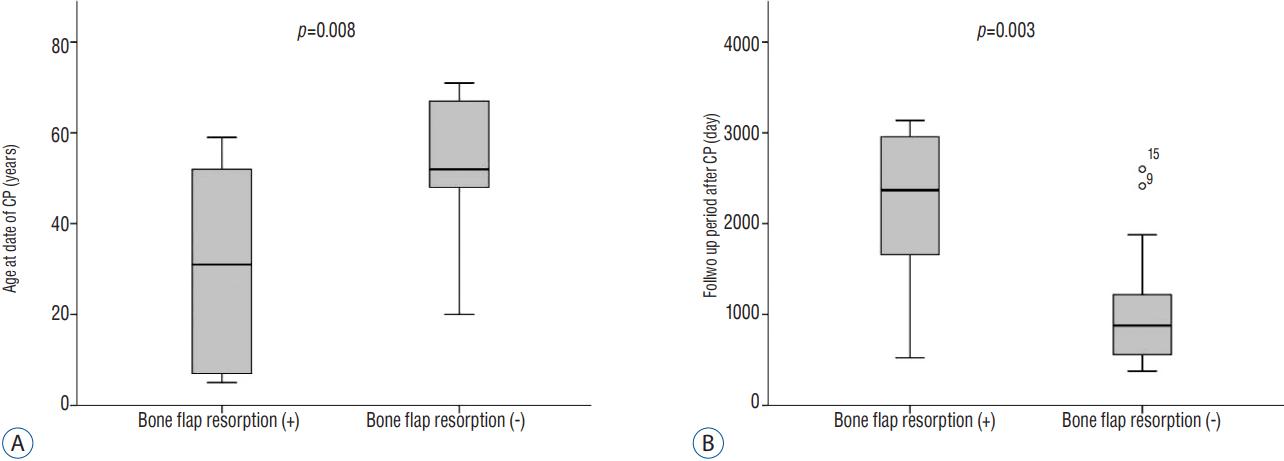 | Fig. 4Box plot showing bone flap resorption related to the age at the date of CP (A) and bone flap resorption related to follow up period after CP (B). CP: cranioplasty. |
Table 2
Univariate analysis of categorical variables between BFR and no-BFR groups
Table 3
Univariate analysis of continuous variables between BFR and no-BFR groups
Go to : 
DISCUSSION
This study showed that the degree of BFR following autologous bone cranioplasty was associated with younger age and longer follow-up period. Furthermore, the incidence and degree of BFR were found to be 27.6% and 2.7%/year after cranioplasty, respectively.
As previously mentioned, the incidence of BFR varies from study to study. These variations in BFR may be attributed to different BFR definitions and follow-up durations. Dünisch et al.7) measured defect size using the formula A=π/4×B×b (with B and b being the longest diameters of an elliptical area) and classified the degree of BFR as either type I necrosis (a thinning of the bone flap and/or a beginning resorption along the rims of the flap) or type II necrosis (as a circumscribed, complete lysis of the bone within the flap, including inner and outer table). Another investigator calculated defect size as an ellipse with π×a×b (longest axis being 2a and the 90° short axis being 2b)18). Because these methods are semi-quantitative and BFR has an irregular margin, we quantitatively measured the decrement ratio of the bone flap size using the ROI curve. This method is quantitative and superior in calculating the BFR rate after cranioplasty.
Previous studies suggest younger age, bone flap fragmentation, shunt-dependent hydrocephalus, large bone defect, and time interval from craniectomy to cranioplasty are the risk factors of BFR3,6,19). Younger age was also indicated as a risk factor of BFR in our study. Although the exact mechanism relating to this remains unknown, a thinner skull and faster bone turn-over may be associated with BFR in the younger poulation2,7,12,16,17,19).
Bone flap fragmentation has also been suggested as a risk factor for BFR2,3,19) and could potentially result in severe disruption of the blood circulation, disturbing angiogenesis, and leading to nutritional deficit of the bone flap2,3,8,19). In 2013, Bowers et al.2) showed that comminuted skull fracture was a risk factors for BFR in pediatric patients following cranioplasty. On the contrary, there was no correlation between bone flap fragmentation and BFR in a study of younger patients <19 years of age10). We assumed that comminuted fracture may hinder the bone remodeling process and our study population included only four patients <19 years, therefore we did not investigate correlation between comminuted fracture and BFR. Instead, we investigated the association between linear skull fracture and BFR and found no statistically significant difference.
Shunt-dependent hydrocephalus as a risk factor for BFR is controversial. Some studies suggest that shunt placement reduces adherence of the dura mater to the bone flap and negatively influences skull growth, thus contributing to BFR2,7,10,15,19). However, in agreement with our finding, Brommeland et al.3) failed to demonstrate correlation between shunt-dependent hydrocephalus and BFR.
Another controversy is the effect of time interval to cranioplasty. Many investigators recommend cranioplasty be performed after 3 months from craniectomy if the neurological status and medical conditions are stabilized4,7). Conversely, delayed cranioplasty can result in complications such as sunken scalp, decreased brain perfusion, and abnormal cerebrospinal fluid dynamics. In a retrospective study of 77 patients with autologous bone flap, longer duration between craniectomy and cranioplasty was associated with BFR3). However, in agreement with our findings, a recent study failed to demonstrate any association between the timing of cranioplasty and BFR7). This discrepancy may be attributed to the heterogeneous and retrospective design of each study.
Recent research has indicated an association between bone flap storage method and BFR5,14). A retrospective study of 290 patients showed that the incidence of BFR was higher in the cryopreservation group compared with the subcutaneous pocket group5). The authors hypothesized that cryopreservation of the bone flap might lead to increased osteoclast activity and bone resorption5). Interestingly, bone flap storage in the abdominal subcutaneous fat is reported to lead to osteolysis by macrophages, which may result in enhanced BFR20). Cryopreserved autologous bone grafts were used in the current study, therefore we could not compare the incidence of BFR according to storage methods.
This study has some limitations. First, this single center study is limited by its small size and retrospective design. Second, two-dimensional measurement of stereoscopic bone flaps may be problematic in determining the exact area. When considering the curvature of skull vault, the degree of BFR can be underestimated at the near the vertex of skull. Third, although the longer the duration of follow-up, the risk of BFR increased, we could not estimate whether every bone flap might undergo resorption or there might be time window for osteoconduction of re-inserted bone flap. Further research will be needed. Nonetheless, we minimized measurement error by calculating the area difference using a program that enabled the ROI free draw method.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download