INTRODUCTION
Traumatic subdural hematoma (SDH) is a common pathological entity in neurosurgery. Between 12% and 29% of patients with severe traumatic brain injury has acute SDH1). The natural course of acute SDH differs among patients. Many patients with small acute SDH and mild neurologic deficit are managed conservatively. However, progression or conversion from acute to chronic SDH is a common cause of clinical deterioration in patients that necessitates surgical treatment8). With the aging of the population, neurosurgeons are faced with a growing number of elderly with acute SDH who are treated with anticoagulant medications. Open craniotomy for immediate hematoma removal is difficult in these patients4). Neurosurgeons should delay surgery in such patients because of the risk of bleeding. Until now, the management of these patients has been unclear.
We retrospectively analyzed patients with acute SDH who underwent delayed burr hole surgery at our institute. The purpose of this study was to evaluate the effectiveness and efficacy of delayed burr hole surgery in relation to the reduction of postoperative SDH volume.
Go to : 
MATERIALS AND METHODS
We retrospectively reviewed and analyzed patients that underwent delayed burr hole operation initially diagnosed as acute SDH at our institute between 2010 and 2012. The scope of the study was traumatic SDH, not spontaneous SDH. All included patients were initially treated conservatively for several reasons including small SDH, old age, or anticoagulant usage. During follow-up, SDH increase or decrease in conscious patients treated by delayed burr hole surgery were analyzed.
Age, sex, Glasgow coma scale (GCS), SDH location, and medical history of anticoagulant agent usage were collected. Laboratory data were included for coagulation parameters. These included prothrombin time, international normalized ratio (INR), platelet function assay and platelet count. Maximum SDH thickness, midline shifts, volume of the SDH (length×width×depth/2) and hounsfield unit (HU) were evaluated by initial and pre-operative brain computed tomography (CT) scanning. Outcomes were length of delayed operation in days, reduction of SDH volume after operation and the Glasgow outcome scale (GOS) score at discharge. Reduction of SDH volume (%) was calculated as ([volume at preoperative SDH-SDH volume after operation]/volume at preoperative SDH×100). Reduction of SDH volume was rated excellent (≥75%), good (≥50%), fair (≥25%), and poor (<25%). The patients were divided two groups according to reduction of volume of SDH also. Reduction of SDH volume of ≥50% or more was defined group A. The other patients were defined as group B. Formal comparisons between two groups were made using independent t-tests. A p-value <0.05 was regarded as statistically significant. All statistical analysis was conducted using Medcalc® (ver. 9.0; Medcalc software, Broekstraat, Belgium).
Go to : 
RESULTS
A total of 378 ASDH patients were admitted at our institute during the follow-up period. Among them, 18 patients were available for this analysis. In most cases, the reason for delayed burr hole operation was a progression of the SDH size with accompanying symptoms, old age and prolongation of coagulation parameters. The 18 patients comprised five women and 13 men. Mean age was 67.5±14.9 years (range, 53–83). Five patients (28%) were taking prescribed anticoagulant medications, which included aspirin, clopidogrel and warfarin. Among them, three patients showed prolongation of coagulation parameters. In 11 patients (61%), SDH was located at right side convexity and at the left side in seven patients (39%). Mean GCS score was 11.1±2.0 (range, 8–14). Patients’ characteristics are described in Table 1. Serial brain CT scans revealed a change in the high density SDH to low density and an increase in volume with a midline shift in the chronic SDH (Fig. 1). The mean delayed operation day was 13.9±7.5 days (range, 7–28 days). Pre-operatively, the mean GCS worsened to 10.6±2.5 (range, 6–14). Eight patients (44%) patients displayed worsened GCS compared to at admission. Maximal thickness of SDH changed from 10.0±3.5 mm to 12.2±3.7 mm. Volume of SDH changed from 38.7±28.0 mL to 42.6±29.6 mL. Midline shifts changed from 5.8±3.3 mm to 6.6±3.3 mm (Fig. 2). HU changed from 66.4±11.2 to 53.2±20.6.
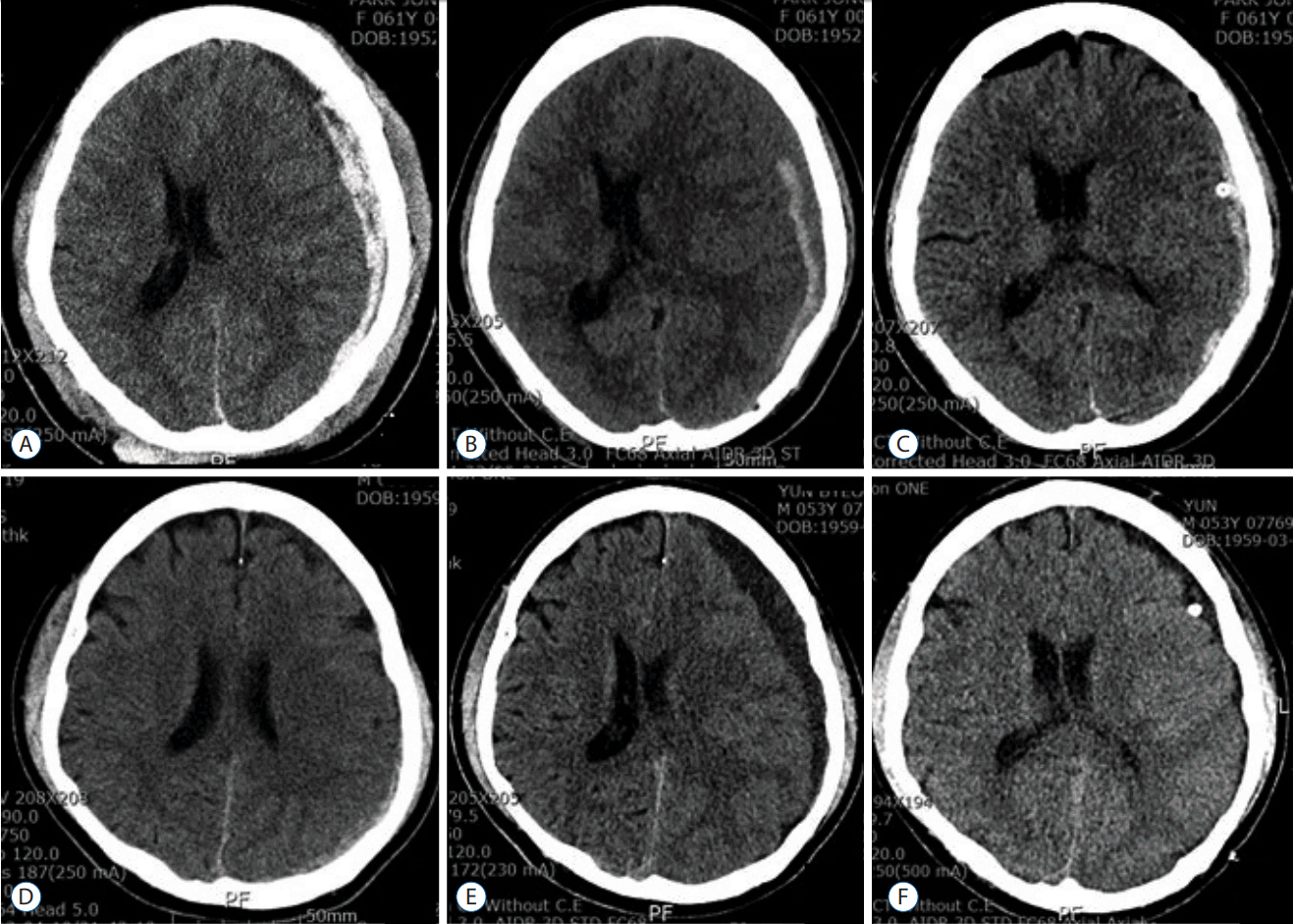 | Fig. 1Data of patients 1 and 14. Patient 1 (female/61): the initial CT image (A) shows a thick hyper-dense ASDH at the left frontotemporoparietal convexity. The follow-up CT (B), which was performed 1 weeks after conservative treatment, reveals a hypodense SDH with an increase of midline shift and volume of SDH. Postoperative CT (C) demonstrates reduction of SDH. Patient 14 (male/53): The initial CT image (D) reveals a thin hyperdense ASDH at the left parietoocipital convexity. The follow-up CT, which was performed 18 days, reveals a hypodense SDH with an increase of midline shift to the right hemisphere. Postoperative CT (F) shows reduction of SDH. CT: computed tomography, ASDH: acute subdural hematoma, SDH: subdural hematoma. |
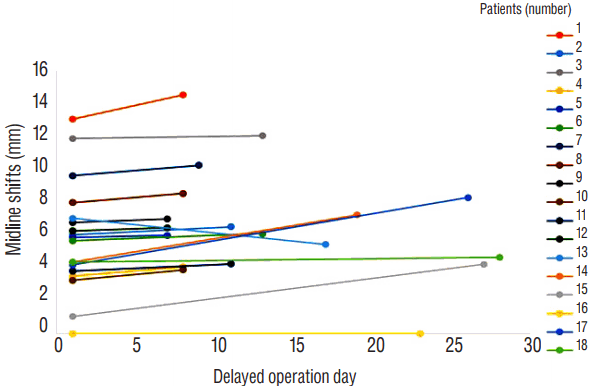 | Fig. 2Before operation, midline shifts were increased from 5.8±3.3 mm to 6.6±3.3 mm. The number of patients were matched to Table 1. |
Table 1
Summary of characteristics of the patients
| No. | Sex | Age | SDH location | Anticoagulant usage | Initial | Delayed operation day | Reduction of SDH volume (%) | GOS at discharge | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCS | HU | Maximal thickness of SDH (mm) | SDH volume (mL) | Midline shift (mm) | ||||||||
| 1 | F | 61 | Lt | - | 12 | 69 | 9.1 | 31.5 | 13.1 | 8 | 86.2 | 1 |
| 2 | M | 30 | Rt | - | 8 | 62 | 5.8 | 21.4 | 6.0 | 11 | 80.3 | 3 |
| 3 | F | 78 | Rt | A+C* | 13 | 78 | 12.3 | 19.2 | 11.9 | 13 | 80.2 | 1 |
| 4 | F | 38 | Rt | C | 9 | 83 | 10.2 | 19.6 | 3.5 | 8 | 70.7 | 2 |
| 5 | M | 80 | Lt | - | 12 | 65 | 18.1 | 111.2 | 5.9 | 7 | 63.7 | 1 |
| 6 | M | 80 | Rt | - | 13 | 75 | 14.1 | 23.2 | 5.7 | 13 | 68.5 | 1 |
| 7 | M | 71 | Rt | - | 10 | 65 | 11.2 | 33.6 | 9.6 | 9 | 59.9 | 1 |
| 8 | M | 70 | Rt | W† | 12 | 51 | 9.1 | 28.4 | 8.0 | 8 | 61.2 | 1 |
| 9 | M | 83 | Lt | - | 9 | 51 | 11.8 | 40.1 | 6.8 | 7 | 50.1 | 2 |
| 10 | M | 72 | Rt | - | 11 | 77 | 5.7 | 113.7 | 3.3 | 8 | 52.2 | 1 |
| 11 | M | 79 | Lt | A‡ | 13 | 75 | 11.9 | 37.1 | 3.8 | 11 | 40.3 | 2 |
| 12 | M | 74 | Lt | A | 8 | 81 | 11.5 | 32.4 | 6.3 | 7 | 42.6 | 2 |
| 13 | M | 61 | Rt | - | 10 | 79 | 9.1 | 38.7 | 7.0 | 17 | 37.4 | 1 |
| 14 | M | 53 | Lt | - | 12 | 55 | 5.5 | 18.8 | 4.4 | 19 | 35.7 | 1 |
| 15 | M | 76 | Rt | - | 8 | 63 | 3.0 | 32.1 | 1.1 | 27 | 27.4 | 3 |
| 16 | F | 76 | Rt | - | 14 | 63 | 9.6 | 44.8 | 0.0 | 23 | 19.3 | 1 |
| 17 | M | 57 | Lt | - | 12 | 49 | 11.0 | 20.2 | 4.2 | 26 | 13.6 | 2 |
| 18 | M | 76 | Rt | - | 13 | 56 | 11.7 | 24.5 | 4.4 | 28 | 48.8 | 1 |
Post-operatively, the reduction of volume of SDH was 52.1±21.1%. Three patients (17%) displayed excellent results (≥75%), seven patients (39%) good results (≥50%), six patients (33%) fair results (≥25%), and two patients (11%) poor results (<25%). Eleven patients (61%) had a GOS score of 1 (good recovery), five patients (28%) had a GOS score of 2 (moderate disability), and two patients (11%) had a GOS score of 3 (severe disability) at discharge.
Ten patients (56%) were enrolled in group A (≥50%, reduction of SDH volume) and eight patients (44%) were enrolled group B. Mean reduction of SDH volume (%) of group A was 67.3 (±12.2). Initial and pre-operative maximal thickness of SDH and SDH volume was greater in group A than group B. Length of delay of surgery of group A was shorter than group B (9.2±2.3 vs. 19.8±7.7 days; p<0.0008). Midline shifting was greater in group A than group B (7.4±3.3 vs. 3.0±2.4 mm; p<0.02). These results are summarized in Table 2.
Table 2
Summary of characteristics of patients
Go to : 
DISCUSSION
SDH can be divided into acute (<3 days), sub-acute (3–21 days), and chronic (>21 days) according to the time of the injury and the onset of clinical symptoms2,6). Many chronic cases progress from the acute stage7). Blood in the subdural space causes an inflammatory reaction. Within a few days, fibroblasts invade the clot and form a new membrane on the inner and outer surface, followed by growth of new capillaries, enzymatic fibrinolysis and dissolution of the blood clot. Fibrin degradation products are reintegrated into new clots and inhibit hemostasis2,7). Chronic SDH arises due to re-hemorrhage to the hematoma cavity with the osmotic gradient, generation of plasma effusion from the torn arachnoid membrane and the reabsorption of fluid2,7).
Three to 26% of conservatively managed acute SDH patients developed the chronic form, which requires evacuation of the hematoma. These patients may require surgery as early as 11–20 days after the initial injury or as late as 3–7 months7–9).
Rust et al.11) reported the risk of developing chronic SDH was at least 42.5 times higher in patients who were taking warfarin and also increased for patients on aspirin, although this latter risk could not be quantified. Interestingly, Laviv and Rappaport7) reported that the operation rate was significantly higher in patients with a medical history of ischemic heart disease or hypertension, with a 4-fold increase in the risk for developing surgical chronic SDH in patients with ischemic heart disease and a 6-fold increased risk in patients with hypertension. Torihashi et al.12) reported that among 343 surgical cases of chronic SDH, 61 patients experienced a recurrence of the chronic condition. Although anticoagulant therapy had no significant effect on recurrence of chronic SDH, the time interval between the injury and the first operation for patients with antiplatelet and/or anticoagulant therapy was shorter than that for patients not receiving these therapies (29.9 vs. 44.2 days).
There have been few reports of delayed burr hole surgery involving acute SDH patients. Izumihara et al.3) reported that eight acute SDH patients required surgery in the sub-acute and chronic stage. Among them, six patients (75%) underwent burr hole surgery and mean time of delayed surgery was 20 days (range, 16–23). Godlewski et al.2) reported on operative treatment of a series of 100 patients with SDH. Among them, 50 patients (50%) underwent burr hole surgery after sub-acute (n=15, 30%) or chronic stage (n=35, 70%). Mathew et al.10) reported on 15 patients who required delayed burr hole evacuation of their acute SDH. The mean time between initial injury and operation was 14 days (range, 11–20 days). Kim et al.5) reported on delayed surgical evacuation for initially non-operative acute SDH. Sixty four patients (65%) were treated with conservative management and 34 patients (35%) required delayed hematoma evacuation of median of 17 days (range, 6–39 days). Twenty two patients (65%) required surgical intervention within 3 weeks of head injury (sub-acute stage) and the remaining 12 patients (35%) underwent burr hole surgery more than 3 weeks after injury (chronic stage). The authors reported that two prognostic factors, including the initial volume of the ASDH and the degree of midline shift on the initial CT scan, were independently associated with delayed hematoma evacuation for ASDH5).
Delayed mean operation day of our series was 13.9 days (range, 7–28 days), similar to the aforementioned studies. In view of SDH reduction of after operation, patients with greater post-operative reduction of SDH volume (group A) experience a shorter delay of surgery (9.2±2.3 days) than group B (19.8±7.7 days). Midline shifting, maximal thickness of SDH and SDH volume were greater in group A than group B. We think that more shorter delay operation day in group A than group B is influenced by more larger SDH in group A. Delayed burr hole surgery is generally effective and successful in chronic stage. Our series show that among well selected patients, delayed burr hole surgery in patients with acute SDH may be effective for reduction of SDH volume at late sub-acute stage and chronic stage.
Go to : 
CONCLUSION
We analyzed delayed burr hole surgery in patients with initially presented acute SDH. Among well selected patients, delayed burr hole surgery in such patients may be effective for reduction of SDH volume. However, more studies are needed to conclusively establish the effectiveness and safety of delayed burr hole surgery in patients with acute SDH.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download