Abstract
Objective
Total resection without consecutive postoperative whole brain radiation therapy is indicated for patients with a single or two sites of brain metastasis, with close follow-up by serial magnetic resonance imaging (MRI). In this study, we explored the effectiveness, usefulness, and safety of this follow-up regimen.
Methods
From January 2006 to December 2015, a total of 109 patients (76 males, 33 females) underwent tumor resection as the first treatment for brain metastases (97 patients with single metastases, 12 with two metastases). The mean age was 59.8 years (range 27–80). The location of the 121 tumors in the 109 patients was supratentorial (n=98) and in the cerebellum (n=23). The origin of the primary cancers was lung (n=45), breast (n=17), gastrointestinal tract (n=18), hepatobiliary system (n=8), kidney (n=7), others (n=11), and unknown origin (n=3). The 121 tumors were totally resected. Follow-up involved regular clinical and MRI assessments. Recurrence-free survival (RFS) and overall survival (OS) after tumor resection were analyzed by Kaplan-Meier methods based on clinical prognostic factors.
Results
During the follow-up, MRI scans were done for 85 patients (78%) with 97 tumors. Fifty-six of the 97 tumors showed no recurrence without adjuvant local treatment, representing a numerical tumor recurrence-free rate of 57.7%. Mean and median RFS was 13.6 and 5.3 months, respectively. Kaplan-Meier analysis revealed the cerebellar location of the tumor as the only statistically significant prognostic factor related to RFS (p=0.020). Mean and median OS was 15.2 and 8.1 months, respectively. There were no significant prognostic factors related to OS. The survival rate at one year was 8.2% (9 of 109).
Direct resection is an important treatment for metastatic brain tumors12). Resection of a single metastasis in the accessible brain is a standard treatment2,11,12,18). Surgical resection plus postoperative whole brain radiation therapy (WBRT) has proven to be one of the best management combinations for metastatic brain tumors14).
As the survival of the cancer patients has increased, previously unanticipated complications of WBRT have become apparent and their prevalence has increased; these complications include deterioration of both cognitive function and quality of life1,3,4,9,10,15). Refinements in surgical technique and brain imaging have made real total resection of a metastatic brain tumor conceivable19).
In this study gross total resection without consecutive postoperative WBRT was done for select patients with brain metastasis. We explored the effectiveness, value, and safety of the initial postoperative follow-up with serial magnetic resonance imaging (MRI).
From January 2006 to December 2015, a total of 201 patients underwent tumor resection as the first treatment for brain metastases. Among them, 60 patients underwent consecutive stereotactic radiosurgery (SRS) after the tumor resection due to other co-existing metastasi(e)s, and 2 patients underwent consecutive SRS after the tumor resection due to subtotal resection. Thirty patients underwent WBRT due to subtotal resection, pre-existing meningeal metastasis or other numerous metastasis. The three patient groups were excluded in this investigation.
The number of enrolled patients and resected tumors was 109 and 121, respectively. The patients underwent tumor resection as the first treatment for their metastatic brain tumor(s). Resection was total and no postoperative local treatments were used. The mean age of the 109 patients was 59.8 years (range 27–80) at the time of surgical resection. Seventy-six patients were male and 33 were female. Metastasis was synchronous (n=64) or metachronous (n=23). There was a single metastasis in 97 patients and two metastases in 12. The location of the 121 tumors in the 109 patients was supratentorial (n=98) and in the cerebellum (n=23). The origin of the primary cancers was lung (n=45), breast (n=17), gastrointestinal tract (n=18), hepatobiliary system (n=8), kidney (n=7), others (n=11), and unknown origin (n=3) (Table 1).
Total resection was performed in all cases. Postoperatively, the patients were initially observed with regular clinical and MRI follow-up. Total resection was surgeon-defined based on the microsurgical view of the surgical field, intraoperative tumor marginal biopsy, or prompt postoperative MRI findings in the patients’ medical records and/or radiological images. At surgery, en bloc or capsular resection was performed in 53 patients. En bloc removal was not done in the other 56 patients, with piecemeal type resection performed instead.
Recurrence-free survival (RFS) and overall survival (OS) after the resection of the tumor(s) were determined by Kaplan-Meier analysis based on clinical prognostic factors. OS also used data from the cancer registration office at Chonnam National University Hwasun Hospital. In case of local recurrence and/or development of a new metastasis, management was optimal based on the patient’s clinical situation and the number of metastases needing treatment. SPSS version 20.0 (IBM, Quarry Bay, Hong Kong) was used to demonstrate all statistical analyses. The level of significance was set at p<0.05.
During the follow-up, 85 patients (78%) with 97 tumors received serial MRI scans. Fifty-six tumors showed no recurrence without any adjuvant local treatment, representing a numerical tumor recurrence-free rate of 57.7%. The mean and median RFS was 13.6 and 5.3 months, respectively. Kaplan-Meier analysis determined a mean and median RFS of 63.6±7.3 and 22.9 months, respectively (Fig. 1). Kaplan-Meier analysis revealed significantly longer mean RFS of supratentorial tumors compared to infratentorial tumors (71.7±8.3 vs. 13.3±4.3 months, p=0.02). Synchronously developed metastasi(e)s were better controlled than metachronous metastasi(e)s (73.1±10.8 vs. 39.7±5.9 months), but not statistically significantly (p=0.290). Contrary to our expectation, en bloc resected tumors showed shorter RFS than tumors resected piecemeal (49.0±7.3 vs. 63.5±10.7 months); the difference was not statistically significant (p=0.769). Of the primary cancer types, RFS of tumors originated in the lung (60.1±7.8 months) was superior to others (51.0±10.2 months), but the difference was not statistically significant (p=0.086) (Fig. 2, Table 2).
Local progression of the tumors after resection was observed in 36 patients (42.3%). They were managed by SRS (n=18), WBRT (n=5), and repeat surgery (n=3). For the remaining 10 patients, only simple palliation was done considering their poor general condition or very short remaining expected survival. New metastasis during follow-up was observed in 34 patients (40.0%). They underwent SRS (n=21), WBRT (n=3), repeat surgery (n=3), and simple palliation (n=7).
The mean and median OS of the 109 patients was 15.2 and 8.1 months, respectively. Kaplan-Meier analysis determined a mean and median OS of 18.5±2.9 and 8.1±0.8 months, respectively (Fig. 3). The survival rate was 8.2% (9 out of 109) at January 2017, one year after the last patient had been registered. Four patients (3.6%) died due to acute postoperative complications of pneumonia or sepsis. One small cell lung cancer brain metastasis patient showed leptomeningeal and ventricular seeding 3 months after the surgery, but underwent only simple palliative management due to the patient’s low performance. He died 5 months after the surgery. A patient with metastatic breast cancer showed meningeal metastasis near the resected tumor 3 months after the surgery. Therefore, the cause of death was related to resected brain metastasis in 2 patients. The nine deaths were related to brain metastasis directly or indirectly, but the related metastasi(e)s were newly-developed. Consequently, the brain-related death rate of all patients was low (10.1%, 11 of 109).
Kaplan-Meier analysis revealed no statistically significant prognostic factors related to the survival after the resection. Median OS of patients whose primary cancer was lung cancer (8.1±1.3 months) was similar to those with cancers of other origin (7.8±1.4 months, p=0.162). The survival of metachronous metastasis patients (8.7±1.5 months) was non-significantly longer than synchronous metastasis patients (7.5±0.7 months, p=0.098). The median survival of younger patients (age≤65 years; 9.0±1.1 months) had longer survival than that of older patients (age>65 years; 6.9±0.7 months), but the difference was not significant (p=0.331). The mean survival of patients with resection of a single metastasis (19.1±3.4 months) was similar to that of patients with resections of multiple metastases (17.1±3.7 months), but was not significant (p=0.691). Patients with en bloc resected tumor(s) (10.2±1.6 months) showed longer median RFS than the tumors resected piecemeal (7.2±0.7 months), without statistical significance (p=0.128). The mean OS of the patients with supratentorial tumors (20.2±3.7 months) was longer than the patients with infratentorial tumors (13.7±3.0 months), and was not statistically significant (p=0.581) (Fig. 4, Table 3).
Direct resection is still an important significant treatment for brain metastasisrole12). Resection of a symptomatic single metastasis in the accessible brain is considered the standard treatment in the patients with good functional status2,11,12,18). Only direct resection can recover a patient’s neurological deterioration and increased intracranial pressure promptly and continuously, compared to steroid therapy, SRS, or WBRT.
Resection and postoperative WBRT is a good combination therapy in the patients with single metastasis and good performance14). In this setting, WBRT is suited for treatment of microscopic peripheral metastasis remaining after gross total resection, for possible tumor cell seeding that occurred during surgery, and/or for other radiologically invisible but microscopically inoculated metastasi(e)s at other sites considering the pathomechanism with hematogenous metastasis.
However, WBRT could be related to and might even induce the deterioration of the cognitive function and quality of life, although failure of tumor control might be the cause1,3,4,9). Among long-term survivors with brain metastasis, half of the patients demonstrated neuropsychological impairment at 2 years after WBRT10,15). Survival of cancer patients is on the rise. These are expected to include patients with brain metastases. Therefore, we should consider the meaningful long-term survival of cancer patients after treatment for brain metastasis and try to minimize the deterioration of cognitive function and quality of life in the ensuing years.
The second reason of reserving WBRT is the appropriate timing of the approach. Considering the longer survival of brain cancer patients, second and third episodes of metastasis can occur, which may need WBRT. Present, new metastases were detected in 40% of the patients, even though they had one or more sites of metastasis at the initial diagnosis. The new sites were managed in a timely manner using WBRT in our patients. WBRT is typically applied to patients with brain metastasis only once; repeat WBRT is rare. Therefore, WBRT should be done as late as possible, with the knowledge that withholding treatment too long is dangerous.
Recent decades have witnessed refinements in surgical techniques and the introduction of high-resolution MRI. High resolution operative microscopy, neuronavigation, intraoperative marginal frozen biopsy, and microscopic total resection with additional marginal resection19), real total resection of a metastatic brain tumor been realized. High-resolution MRI of the brain can detect even a tiny metastasis, which has decreased the rate of the so called ‘hidden’ metastasis. In the present study, intraoperative cancer cell seeding was rare. Only two patients showed ventricular seeding and/or meningeal metastasis near the resected tumor. However, the patients could have undergone WBRT at the time of identification of tumoral seeding, if their general condition and primary cancer status had been good (Fig. 5).
The results of this study could be favorable. The mean and median OS from the surgery was 457 and 244 days, respectively. Postoperative mortality rate was 3.6%, and the suspected brain-related death was 10.1%. The mean and median RFS of the resected tumors was 409 and 159 days, but numerical recurrence-free rate was 57.7%. Therefore, the local control rate was not favorable, but the management of local recurrence was early and timely. The different types of management were selected according to the patient’s general status, primary cancer status, and development and the number of new metastasis. Thorough and regular clinical and imaging follow-up was mandatory.
Concerning the clinical prognostic factors related to RFS and OS, only the supratentorial location of the tumors showed favorable RFS as compared with cerebellar tumors (p=0.020). This could be due to the deep and small surgical field of the cerebellum. Although the surgeon believed that total resection had been done, it might have been incomplete due to the hindrance of the small and deep surgical field of cerebellum. Sunderland et al.17) reported that a significant increase of leptomeningeal metastasis with cerebellar tumor removed via a piecemeal approach compared with an en block resection. However, our data showed similar local control according to the methods of tumor removal in both supratententorial and cerebellar tumors. Our presumption of these results is that the piecemeal resection group in this study was a kind of selected group who underwent a gross total resection. Despite the tumor was resected via piecemeal fashion, the surgeons’ judgement was total removal based on several operative and radiological findings mentioned previously. It might be the reason why the results of two groups (en bloc vs. piecemeal resection) was similar.
We found no significant prognostic factors related to OS. However, the similarity between synchronous and metachronous metastasis patients indicates that the survival from the diagnosis of the primary cancer could be longer in the metachronous metastasis group. On the other hand, the similar OS after the resection between single metastasis patients and two metastasis patients may indicate that all tumor resection could lead similar outcome regardless of the number of metastasis in case of single or two metastasi(e)s.
Instead of postoperative WBRT, the use of SRS for the resection cavity has been introduced5,7,8,12,13,16). The combination of resection and postoperative SRS to the resection cavity can decrease local recurrence. However, if the resection cavity is large, the target volume at SRS may increase and the tumor control rate could decrease6,12). Marginal irregularity of the postoperative cavity may reduce the conformity of the radiation, and some confusion in image interpretation due to the postoperative change in MRI may also occur. Although ‘tumor bed SRS’ might be a postoperative adjuvant local adjuvant treatment, our suggestion is observation at first and follow-up in case of total removal of the metastasis. We used SRS for local recurrence (n=18) and newly developed metastasis (n=20) during the follow-up. If we had performed postoperative SRS for resection cavity, most of those patients with new metastasis should have undergone a second SRS (Fig. 6).
During the direct resection of the metastatic brain tumor, physicians could encounter unexpected events or situations, such as severe bleeding, brain swelling, intended subtotal resection or unexpected severe leptomeningeal metastasis in the surgical field. For these reasons, postoperative WBRT is recommended, and was done presently for 30 patients.
The limitation of this study might be the start point of total resection of the metastasis brain tumor(s). It may be due to the surgeon’s subjective decision, but it can also depend on the operation techniques, such as en-block resection or metastatectomy19), intraoperative marginal biopsy, and immediate postoperative MRI. Therefore, the decision for a total resection is an integrative rather than subjective. Lastly, postoperative follow-up is very important. Regular MRI follow-up every 2–3 months after the resection is mandatory, and the patients should be educated to visit the neurosurgeon when neurological symptoms occur.
References
1. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 295:2483–2491. 2006.

2. Barker FG 2nd. Craniotomy for the resection of metastatic brain tumors in the U.S., 1988–2000: decreasing mortality and the effect of provider caseload. Cancer. 100:999–1007. 2004.

3. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 316:401–409. 2016.

4. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 10:1037–1044. 2009.

5. Do L, Pezner R, Radany E, Liu A, Staud C, Badie B. Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys. 73:486–491. 2009.

6. Jagannathan J, Yen CP, Ray DK, Schlesinger D, Oskouian RJ, Pouratian N, et al. Gamma knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 111:431–438. 2009.

7. Karlovits BJ, Quigley MR, Karlovits SM, Miller L, Johnson M, Gayou O, et al. Stereotactic radiosurgery boost to the resection bed for oligometastatic brain disease: challenging the tradition of adjuvant whole-brain radiotherapy. Neurosurg Focus. 27:E7. 20009.

8. Kim PK, Ellis TL, Stieber VW, McMullen KP, Shaw EG, McCoy TP, et al. Gamma knife surgery targeting the resection cavity of brain metastasis that has progressed after whole-brain radiotherapy. J Neurosurg. 105(Suppl):75–78. 2006.

9. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 29:134–141. 2011.

10. Laukkanen E, Klonoff H, Allan B, Graeb D, Murray N. The role of prophylactic brain irradiation in limited stage small cell lung cancer: clinical, neuropsychologic, and CT sequelae. Int J Radiat Oncol Biol Phys. 14:1109–1117. 1988.

11. Modha A, Shepard SR, Gutin PH. Surgery of brain metastases--is there still a place for it? J Neurooncol. 75:21–29. 2005.

13. Nataf F, Schlienger M, Liu Z, Foulquier JN, Grès B, Orthuon A, et al. Radiosurgery with or without a 2-mm margin for 93 single brain metastases. Int J Radiat Oncol Biol Phys. 70:766–772. 2008.

14. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 322:494–500. 1990.

15. Saad S, Wang TJ. Neurocognitive deficits after radiation therapy for brain malignancies. Am J Clin Oncol. 38:634–640. 2015.

16. Soltys SG, Adler JR, Lipani JD, Jackson PS, Choi CY, Puataweepong P, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 70:187–193. 2008.

Fig. 1
Kaplan-Meier curve of mean recurrence-free survival (RFS) for the 97 resected tumors of 85 patients.
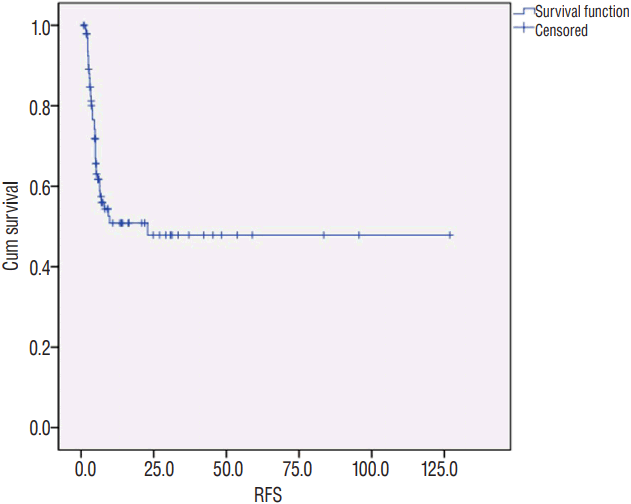
Fig. 2
Kaplan-Meier analysis for prognostic factors related to recurrence-free survival (RFS). A: Tumor location. B: Time of metastasis. C: Surgical method. D: Origin cancer.

Fig. 4
Kaplan-Meier analysis for prognostic factors related to overall survival. A: Age. B: Origin cancer. C: Time of metastasis. D: Surgical method. E: Tumor location. E: Number of metastasis.
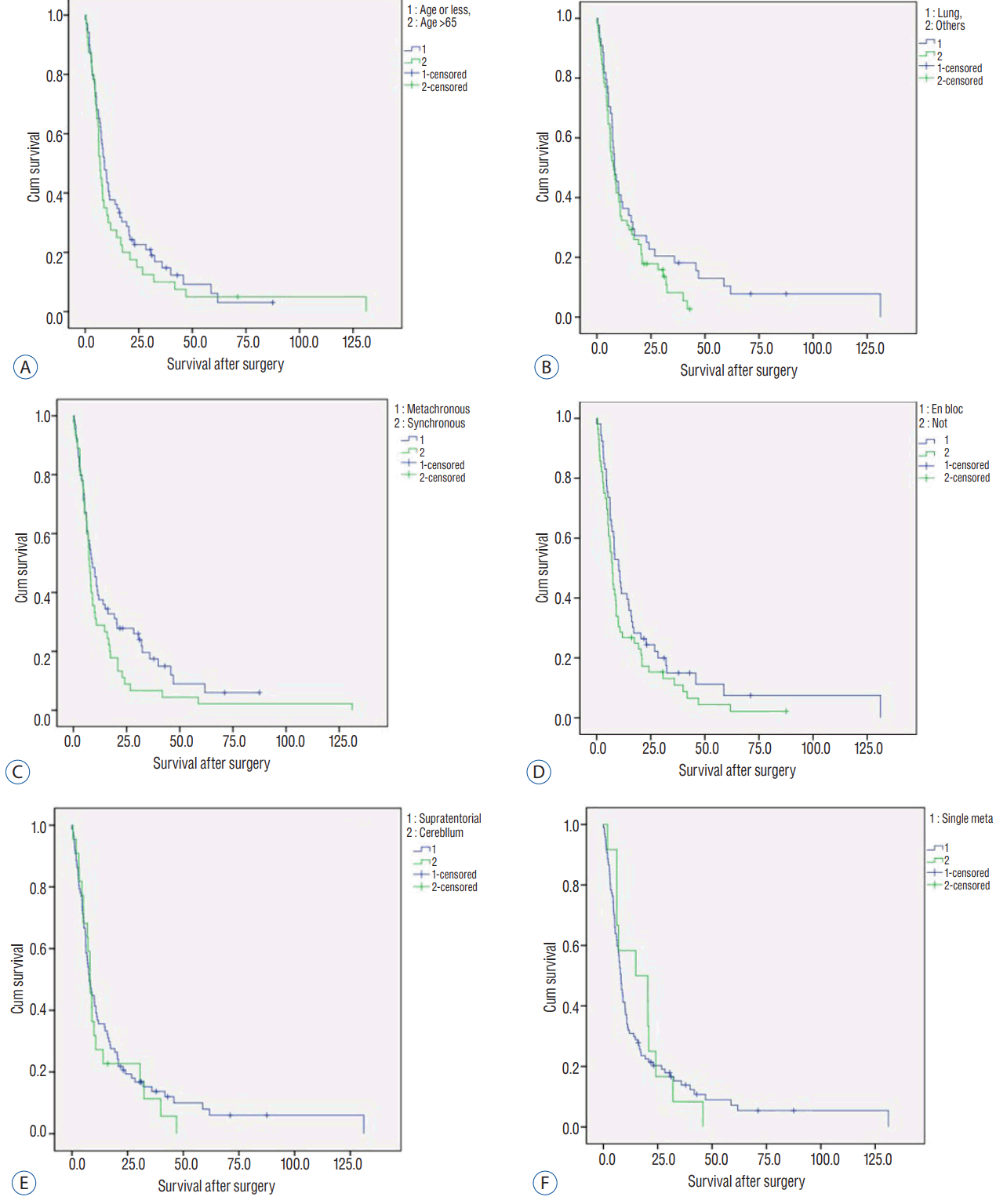
Fig. 5
Illustrative case 1. A 58-year-old male patient with nonsmall cell lung cancer, who underwent tumor resection and planned observation. Preoperative Gadolinium-enhanced magnetic resonance imaging (MRI) shows an enhanced mass in the left parietal lobe (A). Postoperative 3 months follow-up MRI (B) shows a post-resection cavity without local recurrence, and both 1-year (C) and 4-year follow-up (D) MRIs show no local recurrence.
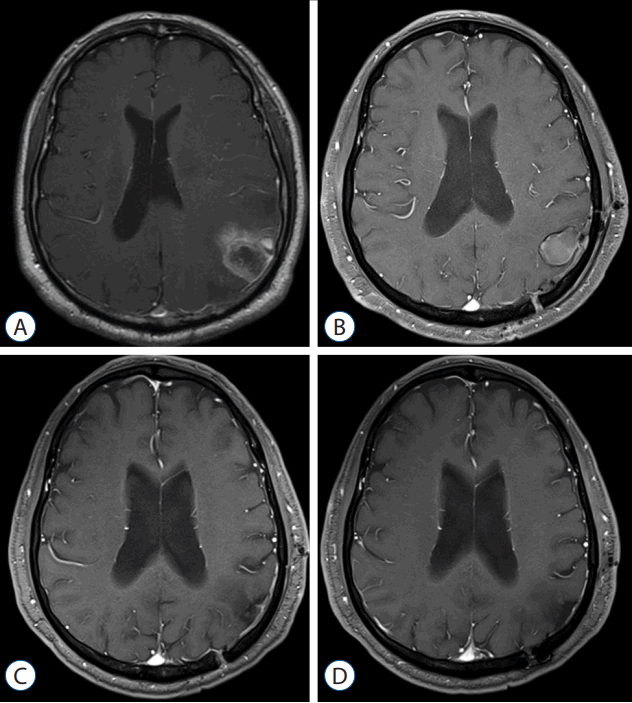
Fig. 6
Illustrative case 2. A 62-year-old female patient with breast cancer, who underwent tumor resection for two tumors and planned observation with regular magnetic resonance imaging (MRI) follow-up. Preoperative Gadolinium-enhanced MRI shows well-enhanced two mass lesions in bilateral frontal lobes (A). Postoperative 3-month follow-up MRI shows post-resection cavities without local recurrence (B). However, both local recurrences and new metastasis (arrow) are observed 6 months after the tumor resection. The patient underwent stereotactic radiosurgery (SRS) for these 3 lesions (C and D). Six months after the SRS, those three lesions were controlled, but, new metastases were observed (E–H), and the patients underwent whole brain radiation therapy (WBRT). Three months after the WBRT, the metastases were all controlled (I–L).
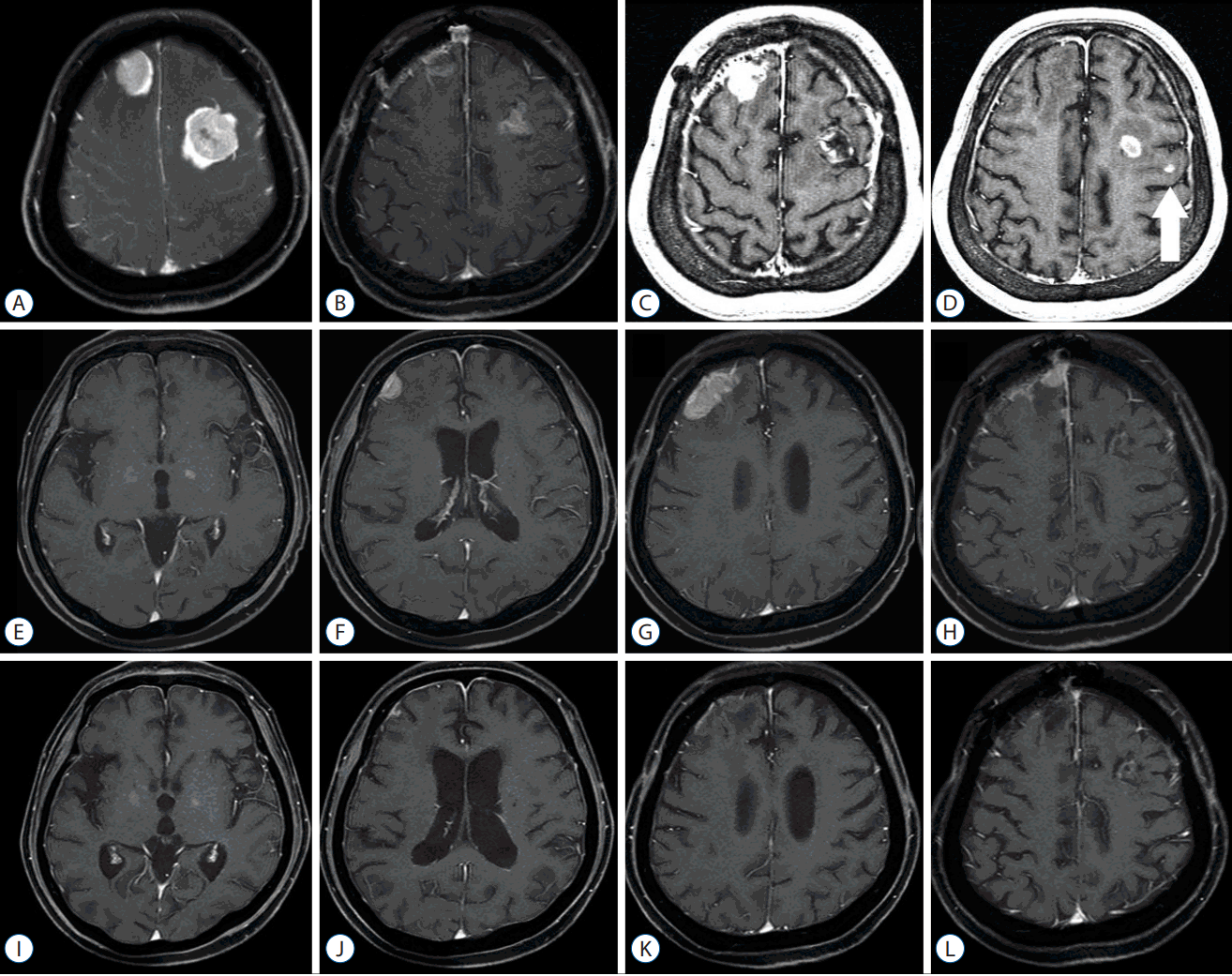
Table 1
Summary of the 109 patients who underwent resection and observation for brain metastasis without prompt postoperative radiation therapy
Table 2
Expected median RFS according to the prognostic factors among the 97 resected tumors of 85 patients who underwent clinical and radiological follow-up
| RFS (months) | p-value* | |
|---|---|---|
| Tumor locations | 0.020 | |
| Supratentorial | 71.7±8.3 | |
| Infratentorial | 13.3±4.3 | |
|
|
||
| Time of metastasis | 0.290 | |
| Synchronous | 73.1± 10.8 | |
| Metachronous | 39.7 ± 5.9 | |
|
|
||
| Surgical methods | 0.769 | |
| En bloc resection | 49.0±7.3 | |
| Piecemeal resection | 63.5±10.7 | |
|
|
||
| Origin cancer | 0.086 | |
| Lung | 60.1±7.8 | |
| Others | 51.0±10.2 | |
Table 3
Expected median OS according to the prognostic factors among the 109 patients
| OS (months) | p-value* | |
|---|---|---|
| Age (years) | 0.331 | |
| ≤65 | 9.0±1.1 | |
| >65 | 6.9±0.7 | |
|
|
||
| Origin cancer | 0.162 | |
| Lung | 8.1±1.3 | |
| Others | 7.8±1.4 | |
|
|
||
| Time of the metastasis | 0.098 | |
| Synchronous | 7.5±0.7 | |
| Metachronous | 8.7±1.5 | |
|
|
||
| Surgical methods | 0.128 | |
| En bloc resection | 10.2±1.6 | |
| Piecemeal resection | 7.2±0.7 | |
|
|
||
| Tumor locations | 0.581 | |
| Supratentorial | 20.2±3.7 | |
| Infratentorial | 13.7±3.0 | |
|
|
||
| Number of metastasis | 0.581 | |
| Single | 19.1±3.4 | |
| Multiple | 17.1±3.7 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download