This article has been retracted. See "Minimally Invasive Anterior Decompression Technique without Instrumented Fusion for Huge Ossification of the Posterior Longitudinal Ligament in the Thoracic Spine : Technical Note And Literature Review" in Volume 62 on page 618.
Abstract
Objective
Several surgical methods have been reported for treatment of ossification of the posterior longitudinal ligament (OPLL) in the thoracic spine. Despite rapid innovation of instruments and techniques for spinal surgery, the postoperative outcomes are not always favorable. This article reports a minimally invasive anterior decompression technique without instrumented fusion, which was modified from the conventional procedure. The authors present 2 cases of huge beak-type OPLL. Patients underwent minimally invasive anterior decompression without fusion. This method created a space on the ventral side of the OPLL without violating global thoracic spinal stability. Via this space, the OPLL and anterior lateral side of the dural sac can be seen and manipulated directly. Then, total removal of the OPLL was accomplished. No orthosis was needed. In this article, we share our key technique and concepts for treatment of huge thoracic OPLL.
Methods
Case 1. 51-year-old female was referred to our hospital with right lower limb radiating pain and paresis. Thoracic OPLL at T6–7 had been identified at our hospital, and conservative treatment had been tried without success. Case 2. This 54-year-old female with a 6-month history of progressive gait disturbance and bilateral lower extremity radiating pain (right>left) was admitted to our institute. She also had hypoesthesia in both lower legs. Her symptoms had been gradually progressing. Computed tomography scans showed massive OPLL at the T9–10 level. Magnetic resonance imaging of the thoracolumbar spine demonstrated ventral bony masses with severe anterior compression of the spinal cord at the same level.
Results
We used this surgical method in 2 patients with a huge beaked-type OPLL in the thoracic level. Complete removal of the OPLL via anterior decompression without instrumented fusion was accomplished. The 1st case had no intraoperative or postoperative complications, and the 2nd case had 1 intraoperative complication (dural tear) and no postoperative complications. There were no residual symptoms of the lower extremities.
Conclusion
This surgical technique allows the surgeon to safely and effectively perform minimally invasive anterior decompression without instrumented fusion via a transthoracic approach for thoracic OPLL. It can be applied at the mid and lower level of the thoracic spine and could become a standard procedure for treatment of huge beak-type thoracic OPLL.
Ossification of the posterior longitudinal ligament (OPLL) is commonly reported in Asian populations21). OPLL may involve the cervical and thoracic spine and could cause severe myelopathy5). Conservative treatment is ineffective to treat thoracic myelopathy. Despite several surgical techniques that have been reported for treatment of thoracic OPLL, satisfactory results are not always achieved. Thus far, there are no standard treatment protocols established for thoracic OPLL4). Direct removal of the massive OPLL is the most valid method of relieving anterior spinal cord compression10,19,22,24). However, in anterior decompression, the compressed spinal cord is quite vulnerable to injury during removal of OPLL23). Postoperative neurological deterioration and complications have been reported in several articles3,15). In conventional anterior decompression and fusion, the patients were confined to bed for 3 weeks3). Anterior instrumentation might be needed to maintain stability. A hard orthosis was used postoperatively3). In this technical note, we present two cases using our novel technique to remove OPLL, which is a relatively safe and minimally invasive procedure.
This 51-year-old female was referred to our hospital with right lower limb radiating pain and paresis. Sphincter tone had decreased. Her symptoms had gradually progressed over the previous 2 months. Thoracic OPLL at T6–7 had been identified at our hospital, and conservative treatment had been tried without success. Her medical history included hypertension.
After admission to our hospital, the patient complained of difficulty walking with bilateral lower extremity weakness. She depended on a wheelchair. Physical examination revealed hyperreflexia at the bilateral knee and ankle joints. As a measure of thoracic myelopathy, her preoperative Japanese Orthopedic Association (JOA) score was 9 (total score of 17). Myelography, computed tomography (CT) scanning, and magnetic resonance imaging (MRI) revealed an isolated huge beak-type OPLL at the T6–7 level with severe spinal cord compression (Fig. 1) and mild C4–5 spinal stenosis.
After the operation, the patient’s preoperative neurological symptoms gradually improved. No spinal orthosis was used. One week after surgery, the patient could walk using a cane. Postoperative CT and MRI revealed complete removal of the OPLL (Fig. 2). After 6 months, the patient’s preoperative neurological symptoms had improved. His postoperative JOA score was 14 (total score of 17).
This 54-year-old female with a 6-month history of progressive gait disturbance and bilateral lower extremity radiating pain (right>left) was admitted to our institute. She also had hypoesthesia in both lower legs. Her symptoms had been gradually progressing.
A manual muscle test revealed a tibialis anterior score of 5/5 in right side, 2/5 in left side and an extensor hallucis longus score of 5/5 in right side, 2/5 in left side, particularly in the ankle dorsiflexors because of a past left ankle fracture. As a measure of thoracic myelopathy, her preoperative JOA score was 10 (total score of 17). CT scans showed massive OPLL at the T9–10 level. MRI of the thoracolumbar spine demonstrated ventral bony masses with severe anterior compression of the spinal cord at the same level (Fig. 3).
Immediately after surgery, this patient’s preoperative lower extremity radiating pain had improved. Ten days after the operation, the patient could walk using a cane. Postoperative CT and MRI revealed total removal of the OPLL (Fig. 4). After 4 months, her preoperative gait disturbance had improved, and she could walk without a cane. Her postoperative JOA score was 14 (total score of 17).
Because of massive OPLL with spinal cord compression and a kyphotic thoracic spine, posterior laminectomy or laminoplasty might not provide enough decompressive effect. Anterior decompression via a posterior approach was very difficult because of inaccessibility to the surgical field of view. Therefore, we decided to perform a minimally invasive anterior decompression technique without instrumented fusion via transthoracotomy for thoracic OPLL under intraoperative monitoring using motor evoked potentials and somatosensory evoked potentials.
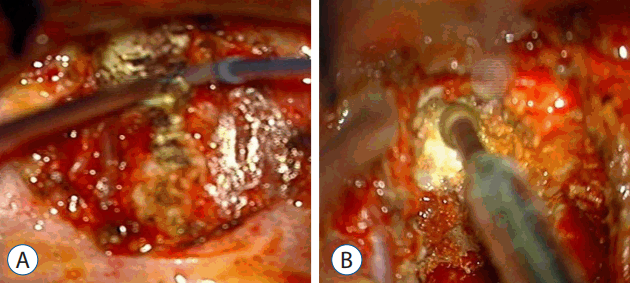
The patients were placed in the decubitus position with the dominant side of the OPLL up. An anterior lateral approach via thoracotomy was used. The index disc was exposed and confirmed by fluoroscopy. The rib head was excised, and the pedicle below the index level was exposed (Fig. 5A).
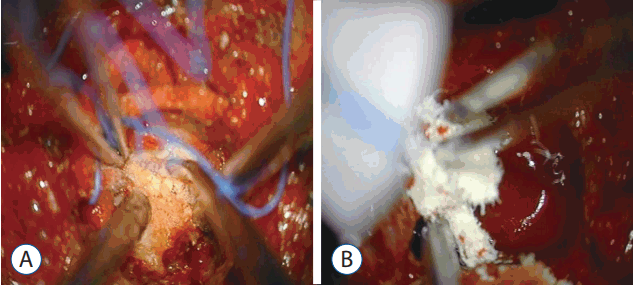
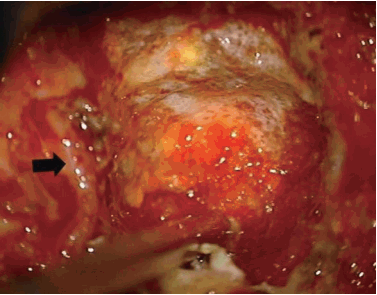
Two pedicles (upper and lower body) and the posterior margin of the disc were identified under a microscope. The posterior half of the disc was removed. Additionally, ostectomy was simultaneously performed with a high speed burr. The area included the partial posterior vertebral body of the inferior upper vertebra, superior lower vertebra, and total portion of the pedicle in the lower vertebra. The partial vertebral body ostectomy provided space for the ventral side of OPLL. The pedicle ostectomy (Fig. 5B) provided more space to deal with the adhesion between the OPLL and the dura. Via this space, the OPLL and the dural sac could be seen directly. The OPLL mass was floated by drilling delicately. After releasing the adhesion between the ossified ligament and the ventral aspect of the dural sac, the OPLL was removed completely (Fig. 6A). Avitene and cottonoids were used for bleeding control between the OPLL mass and the dural sac (Fig. 6B). During the operation, the upper and lower segmental arteries were preserved, and the nerve root was always protected through the whole procedure (Fig. 7). Unfortunately, there was a dural tear and cerebrospinal fluid (CSF) leakage in the 2nd case, so a Lyoplant patch was inserted in the subdural area followed by Tachosil patch application. The chest tube was kept in the operating room after the operation. After 2 weeks drainage, drainage was reduced and CSF leakage was not observed. And then, chest tube was removed.
We used this surgical method in 2 patients with a huge beaked-type OPLL in the thoracic level. Complete removal of the OPLL via anterior decompression without instrumented fusion was accomplished. The 1st case had no intraoperative or postoperative complications, and the 2nd case had 1 intraoperative complication (dural tear) and no postoperative complications. There were no residual symptoms of the lower extremities.
Decompression surgery is the treatment of choice for compressive myelopathy2). However, the surgical outcomes of thoracic OPLL are not as favorable compared with those of cervical OPLL12). The unfavorable outcome can be explained by anatomical and pathophysiological factors of thoracic OPLL: 1) the natural kyphosis of the thoracic spine may limit the positive effect of posterior decompression; 2) the thoracic spinal cord is relatively avascular, which is more vulnerable during decompressive manipulation11); and 3) the anterior approach is restricted and the technique is demanding. The strong adhesion between OPLL and the dural sac increases the difficulty of removing the OPLL and increases the risk of intraoperative damage. Factors that significantly contributed to surgical outcomes were the combined use of instrumentation and the level of maximum OPLL at the thoracic spine12). Surgical complications can occur early or be delayed. The perioperative complications reported by surgeons include epidural hematoma, CSF leakage, infection, and neurological deterioration15). The real causes of early neurological deterioration are not well known.
The surgical management of thoracic OPLL is challenging, even for experienced surgeons. Surgical management for OPLL includes several options: 1) posterior decompression with or without instrumentation; 2) laminoplasty; 3) posterior decompression with instrumented fusion then 2nd stage anterior decompression; 4) anterior decompression via a posterior approach; and 5) anterior decompression via an anterior approach. A multiple institutional retrospective study has been performed including 34 hospitals in which detailed data was analyzed on patients who had thoracic OPLL8). The study analyzed the following procedures: beak-type OPLL treated by anterior decompression with instrumented fusion using an anterior approach and laminoplasty; continuous waveform and cylindrical type OPLL treated by laminoplasty; and mixed type OPLL treated by laminectomy with fusion and anterior decompression with fusion via an anterior approach. The results revealed no statistically significant difference in outcomes among the different surgical methods.
Because of lordotic or mild kyphotic curvature at the upper thoracic spine, indirect posterior decompression can be accomplished by laminectomy or extensive laminoplasty12). Although the thoracic spine anchored to the chest cage has limited motion, progressive kyphosis might occur after posterior laminectomy. Some surgeons use posterior segmental instrumentation with fusion for correction of kyphosis or prevention of kyphosis progression. Thus, the posterior decompressive effect can be maintained23). However, favorable outcomes may not be achieved because the OPLL remains and because of the physiological kyphosis of the thoracic spine14,20). The spinal cord has limited dorsal shift in the middle and lower thoracic spine.
Some authors advocate favorable surgical outcomes using the circumferential approach for complete decompression7,19). This approach improves neurological function better than posterior laminectomy alone18). Matsuyama et al. showed that kyphotic reduction in the thoracic spine provides a dorsal shift of the spinal cord by using intraoperative sonography8,14). Posterior decompression may provide posterior space for the spinal cord to escape from injury by anterior manipulation19). Therefore, the advantage of this surgical method was a relatively low risk of spinal cord damage while removing the OPLL via an anterior approach. Tomita et al.19) reported staged circumspinal decompression including a posterior decompression followed by anterior decompression via thoracotomy. They suggested deep gutter drilling in the bilateral vertebral bodies after posterior laminectomy. Then thoracotomy for anterior decompression with partial vertebrectomy, discectomy, removal of OPLL, and interbody fusion were performed. However, there is still the possibility of spinal cord injury in cases of OPLL with extensive involvement in the thoracic spine. The circumspinal approach has not become the standard protocol for thoracic OPLL because combined operation can increase the operation time, surgical stress, technical demands, and anesthesia risk.
Direct removal of the OPLL is the optimal objective of the surgery. Some surgeons have reported relatively favorable surgical outcomes using anterior decompression via a posterior approach1,17). However, Takahata et al.18) reported a relatively high rate of complications, including neurological deterioration in 33% and dural tear in 40% of the patients. Adherence of the OPLL to the ventral side of the dura increases the risk of spinal cord injury and removal difficulty. It was supposed that the complications were related to insufficient space and the operative field of view. Anterior decompression via a posterior approach has several disadvantages: 1) increasing the risk of spinal cord ischemia because the thoracic nerve roots were ligated16) and 2) a larger operative wound and surgical stress7). However, the OPLL was too massive in this case. Anterior decompression via a posterior approach was very difficult due to the inaccessibility of the surgical field.
Anterior decompression via the transthoracic approach is the most logical approach in the treatment of thoracic myelopathy caused by thoracic OPLL3). However, there are relatively high risks of perioperative complications6,23). Postoperative neurological deterioration was reported in several publications3,15). The patients were restricted to bed and equipped with spinal orthosis after operation. Our technique of minimally invasive anterior decompression without instrumented fusion is a modification of the conventional operation. This surgical method has several advantages: 1) it retains the advantages of the conventional anterior approach (for example, visualization of the OPLL and anterolateral side of the dural sac directly and a small operative wound); 2) it provides a wider view at the anterior lateral side of the dural sac to maneuver in the anterior decompression than the posterior approach; 3) no nerve roots were sacrificed; 4) neither anterior instrumentation nor fusion were needed to maintain the stability and structure of the thoracic spine; 5) no destruction of posterior spinal elements; and 6) the patients returned to daily life sooner after the operation7,13).
This technique also had some disadvantages: 1) this method was technically demanding; 2) it is only indicated for beak-type OPLL at one or two consecutive levels; and 3) it is indicated only for the middle and lower thoracic spine9).
By this method, it could be possible to remove the OPLL via a wide field of view with minimal destruction, higher safety, and less blood loss. During removal of OPLL with the dura in view, operative safety could be possible. The favorable therapeutic effects were confirmed by 2-year follow-up after the operation. Despite the limited indications, this technique can be performed in the middle and lower thoracic spine and is particularly suitable for massive beak-type thoracic OPLL.
This surgical technique allows the surgeon to safely and effectively perform minimally invasive anterior decompression without instrumented fusion via a transthoracic approach for thoracic OPLL. It can be applied at the mid and lower level of the thoracic spine and could become a standard procedure for treatment of huge beak-type thoracic OPLL.
Acknowledgements
This study was supported by a grant from the Spine Health Wooridul Hospital. None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
1. Abumi K, Suda K. Surgical strategy for thoracic OPLL. Orthop Surg Traumatol. 45:615–621. 2002.
2. Aizawa T, Sato T, Sasaki H, Matsumoto F, Morozumi N, Kusakabe T, et al. Results of surgical treatment for thoracic myelopathy: minimum 2-year follow-up study in 132 patients. J Neurosurg Spine. 7:13–20. 2007.

3. Fujimura Y, Nishi Y, Nakamura M, Toyama Y, Suzuki N. Long-term follow-up study of anterior decompression and fusion for thoracic myelopathy resulting from ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 22:305–311. 1997.

4. Fujimura Y, Nishi Y, Nakamura M, Watanabe M, Matsumoto M. Myelopathy secondary to ossification of the posterior longitudinal ligament of the thoracic spine treated by anterior decompression and bony fusion. Spinal Cord. 35:777–784. 1997.

5. Ido K, Shimizu K, Nakayama Y, Yamamuro T, Shikata J, Matsushita M, et al. Anterior decompression and fusion for ossification of posterior longitudinal ligament in the thoracic spine. J Spinal Disord. 8:317–323. 1995.

6. Kato S, Kawahara N, Tomita K, Murakami H, Demura S, Fujimaki Y. Effects on spinal cord blood flow and neurologic function secondary to interruption of bilateral segmental arteries which supply the artery of Adamkiewicz: an experimental study using a dog model. Spine (Phila Pa 1976). 33:1533–1541. 2008.

7. Kawahara N, Tomita K, Murakami H, Hato T, Demura S, Sekino Y, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 33:39–46. 2008.

8. Kawakami N, Mimatsu K, Kato F, Sato K, Matsuyama Y. Intraoperative ultrasonographic evaluation of the spinal cord in cervical myelopathy. Spine (Phila Pa 1976). 19:34–41. 1994.

9. Kojima T, Waga S, Kubo Y, Matsubara T. Surgical treatment of ossification of the posterior longitudinal ligament in the thoracic spine. Neurosurgery. 34:854–858. discussion 858. 1994.

10. Kurosa Y, Yamaura I, Nakai O, Shinomiya K. Selecting a surgical method for thoracic myelopathy caused by ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 21:1458–1466. 1996.

11. Lazorthes G, Gouaze A, Zadeh JO, Santini JJ, Lazorthes Y, Burdin P. Arterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathways. J Neurosurg. 35:253–262. 1971.
12. Matsumoto M, Chiba K, Toyama Y, Takeshita K, Seichi A, Nakamura K, et al. Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine: a multi-institutional retrospective study. Spine (Phila Pa 1976). 33:1034–1041. 2008.

13. Matsuyama Y, Sakai Y, Katayama Y, Imagama S, Ito Z, Wakao N, et al. Indirect posterior decompression with corrective fusion for ossification of the posterior longitudinal ligament of the thoracic spine: is it possible to predict the surgical results? Eur Spine J. 18:943–948. 2009.

14. Matsuyama Y, Yoshihara H, Tsuji T, Sakai Y, Yukawa Y, Nakamura H, et al. Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine: implication of the type of ossification and surgical options. J Spinal Disord Tech. 18:492–498. discussion 498. 2005.
15. Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech. 21:116–119. 2008.

16. Murakami H, Kawahara N, Tomita K, Demura S, Kato S, Yoshioka K. Does interruption of the artery of Adamkiewicz during total en bloc spondylectomy affect neurologic function? Spine (Phila Pa 1976). 35:E1187–E1192. 2010.

17. Ohtsuka K, Terayama K, Tsuchiya T, Wada K, Furukawa K, Ohkubo M. A surgical procedure of the anterior decompression of the thoracic spinal cord through the posterior approach. Orthop Surg Traumatol. 26:1083–1090. 1983.
18. Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Minami A. Clinical results and complications of circumferential spinal cord decompression through a single posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine (Phila Pa 1976). 33:1199–1208. 2008.

19. Tomita K, Kawahara N, Baba H, Kikuchi Y, Nishimura H. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine (Phila Pa 1976). 15:1114–1120. 1990.

20. Tokuhashi Y, Matsuzaki H, Oda H, Uei H. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification-kyphosis angle on MRI. Spine (Phila Pa 1976). 31:E26–E30. 2006.
21. Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 184:71–84. 1984.

22. Tsuzuki N, Hirabayashi S, Abe R, Saiki K. Staged spinal cord decompression through posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine (Phila Pa 1976). 26:1623–1630. 2001.

23. Yamazaki M, Mochizuki M, Ikeda Y, Sodeyama T, Okawa A, Koda M, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine (Phila Pa 1976). 31:1452–1460. 2006.

Fig. 1
Preoperative sagittal and axial magnetic resonance (A and B) and computed tomography (C and D) images showing a huge beak-type ossification of the posterior longitudinal ligament at the T6–7 level with severe compression of the spinal cord.

Fig. 2
Postoperative sagittal (A) and axial (B) magnetic resonance and sagittal (C) and axial (D) computed tomography images reveal complete removal of the ossified posterior longitudinal ligament and total decompression of the spinal cord at the T6–7 level.
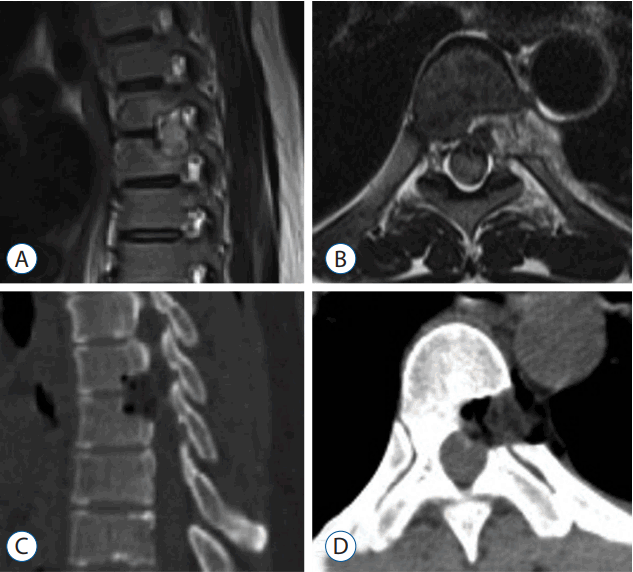
Fig. 3
Preoperative thoracolumbar magnetic resonance (A and B) and computed tomography (C and D) images show the beak-type ossification of the posterior longitudinal ligament at T9–10, which had compressed the spinal cord.
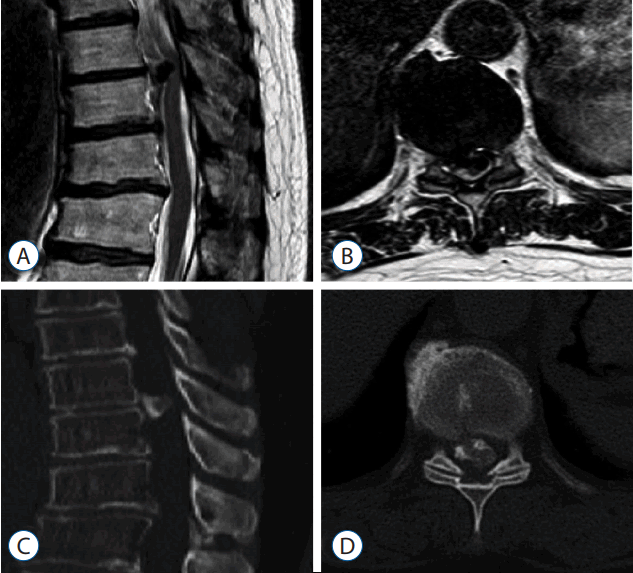
Fig. 4
Postoperative sagittal (A) and axial (B) magnetic resonance and sagittal (C) and axial (D) computed tomography images show complete removal of the ossified posterior longitudinal ligament and total decompression of the spinal cord at the T9–10 level.
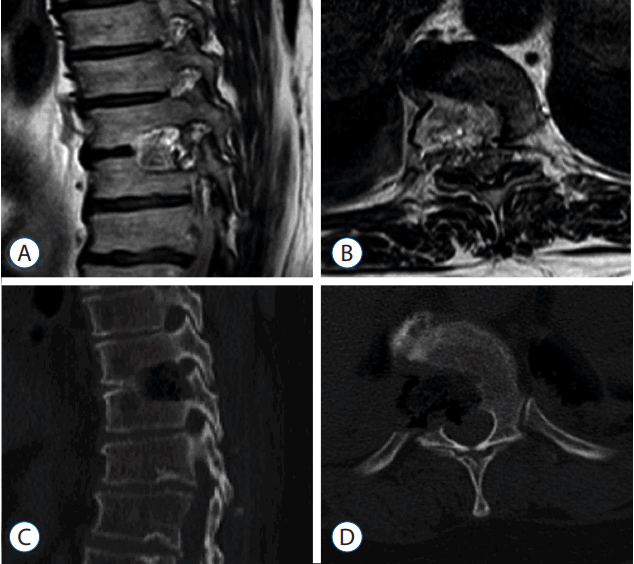
Fig. 5
A: The endothoracic fascia opening can be followed posteriorly to the proximal rib head, which was excised. B: The rostral portion of the pedicle directly caudal to the disc space can also be drilled down to improve exposure of the dural sac and ossification of the posterior longitudinal ligament.