Abstract
Objective
Magnetic resonance imaging (MRI) grading systems using sagittal images are useful for evaluation of lumbar foraminal stenosis. We evaluated whether such a grading system is useful as a diagnostic tool for surgery.
Methods
Between July 2014 and June 2015, 99 consecutive patients underwent unilateral lumbar foraminotomy for lumbar foraminal stenosis. Surgically confirmed foraminal stenosis and the contralateral, asymptomatic neuroforamen were assessed based on a 4-point MRI grading system. Two experienced researchers independently evaluated the MR sagittal images. Interobserver agreement and intraobserver agreement were analyzed using κ statistics.
Results
The mean age of patients (54 women, 45 men) was 62.5 years. A total of 101 levels (202 neuroforamens) were evaluated. MRI grades for operated neuroforamens were as follows: Grade 0 in 0.99%, Grade 1 in 5.28%, Grade 2 in 14.85%, and Grade 3 in 78.88%. Interobserver agreement was moderate for operated neuroforamens (κ=0.511) and good for asymptomatic neuroforamens (κ=0.696). Intraobserver agreement by reader 1 for operated neuroforamens was good (κ=0.776) and that for asymptomatic neuroforamens was very good (κ=0.831). In terms of lumbar level, interobserver agreement for L5–S1 (κ=0.313, fair) was relatively lower than the other level (κ=0.804, very good).
Conclusion
MRI grading system for lumbar foraminal stenosis is thought to be useful as a diagnostic tool for surgery in the lumbar spine; however, it is less reliable for symptomatic L5–S1 foraminal stenosis than for other levels. Thus, various clinical factors as well as the MRI grading system are required for surgical decision-making.
Lumbar foraminal stenosis is caused by the abnormal narrowing of the lateral foramen in the lumbar spinal canal. Lumbar foraminal stenosis is an important pathologic entity to identify in patients being treated for radicular symptoms6,7,10). The intervertebral foramen is bound superiorly and inferiorly by the pedicles of the adjacent vertebra. The anterior boundary is formed by the posterior margin of the vertebral bodies and the intervertebral disc. The posterior boundary is formed of the pars interarticularis, ligamentum flavum, and superior articular process3). The lumbar nerve root can be compressed by components forming boundaries of the intervertebral foramen, such as a herniated disc, hypertrophied facet, and spur from the vertebral endplate2,5,11).
Magnetic resonance imaging (MRI) is widely used in the evaluation of lumbar foraminal stenosis as showing morphological image of the intervertebral foramen. However, there have been few reports on the MRI-based grading or classification of lumbar foraminal stenosis1,4,9,12). Wildermuth et al.12) introduced a grading system based on the degree of epidural fat obliteration. Attias et al.1) suggested a similar classification system according to locations of epidural fat obliteration, but divided the intervertebral foramen into four quadrants. More recently, Lee et al.9) proposed a new grading system that includes the type of stenosis, amount of fat obliteration, and the presence of nerve root compression. However, these reports did not investigate correlations of grades with clinical symptoms and surgical indications. The purpose of this study was thus to evaluate the MRI grading system for lumbar foraminal stenosis in light of clinical data, and to discuss its usefulness as a diagnostic tool for surgery.
All 99 consecutive patients who underwent unilateral lumbar foraminotomy for lumbar foraminal stenosis were studied between July 2014 and June 2015. Surgical decision was made by surgeon’s comprehensive judgment depending on patient’s general condition, patient opinion for treatment process, aspect and degree of symptoms, radiologic findings, and expected postoperative outcome. All patients received sufficient conservative treatment including oral medication, physical therapy, and nerve block before the surgery and if the pain persisted or it did not subside enough despite conservative treatment, surgical treatment was considered. The exclusion criteria were evidence of infection, tumor, trauma, fracture, and/or metabolic neuropathy.
Sagittal T1-weighted imaging was the main method of evaluation, and T2-weighted imaging was also used as an additional tool to exclude false-positive findings that can result from the presence of perineural cysts or nerve root swelling. Surgically confirmed foraminal stenosis (operated neuroforamen) and the contralateral, asymptomatic neuroforamen (non-operated neuroforamen) were assessed based on the 4-point MRI grading system of Lee et al.9). Grade 0 refers to normal neuroforamen. Grade 1 refers to mild foraminal stenosis showing perineural fat obliteration in two opposing directions. Grade 2 refers to moderate foraminal stenosis showing perineural fat obliteration in four directions. Grade 3 refers to severe foraminal stenosis showing morphologic changes in the nerve root (Fig. 1). A comparative example is provided in Fig. 2, which shows MR sagittal images of symptomatic neuroforamen with stenosis (Grade 2) and the contralateral, asymptomatic neuroforamen (Grade 0).
Two experienced researchers (researcher 1, a radiologist; and researcher 2, a neurosurgeon), both of whom had 15 and 20 years of experience, respectively, retrospectively analyzed the MR images of the selected patients. To assess reproducibility, they evaluated the sagittal MRIs independently, and researcher 2 re-evaluated the sagittal MR images after one month. The examinations were reviewed without patient’s clinical data in order to avoid any bias.
A total of 101 levels (202 neuroforamens) in 99 patients, where unilateral lumbar foraminotomy was performed (foraminotomy was performed at two levels in two patients), were analyzed through a combination of both T1- and T2-weighted sagittal images. Symptomatic neuroforamen (operated neuroforamen) and asymptomatic neuroforamen (non-operated neuroforamen) in each level were evaluated.
Interobserver agreement between the two researchers, and intraobserver agreement one month later (researcher 2) were analyzed using linear weighted κ statistics. A κ value of less than 0.20 indicated an interobserver agreement that was poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81 or greater, very good agreement8). The MRI grade of operated neuroforamens and non-operated neuroforamens was compared using an independent t-test. The Statistical Package for the Social Sciences for Windows (version 22.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
The average patient age was 62.6 years (range, 24–86 years) and there were 45 men (45.5%) and 54 women (54.5%). Mean symptom duration until surgical treatment was 8.6 weeks and mean visual analogue scale was 7.7 preoperatively and 1.6 postoperatively (Table 1).
A total of 101 levels (202 neuroforamens) were graded according to Lee et al.’s9) classification. The number of each level was 0 (L1–L2), 5 (L2–L3), 5 (L3–L4), 29 (L4–L5), and 62 (L5–S1) (Table 2).
Operative findings in all cases showed that the nerve root of the symptomatic side was compressed because of several causes: 1) hypertrophic facet (mainly superior facet), 2) marginal end plate osteophytes, 3) disc lesion such as bulging and calcified disc, and 4) thickened ligamentum flavum and foraminal ligaments. The compressed nerve root had edematous aspects and venous congestion around it. These causes were eliminated by decompressive procedure and surgery was finally finished after ensuring that the decompressed nerve root moved freely.
The percentage of each MRI grade for operated/non-operated neuroforamens was as follows: Grade 0, 0.99%/22.77%; Grade 1, 5.28%/57.10%; Grade 2, 14.85%/12.54%; Grade 3, 78.88%/7.59%. The mean MRI grades of operated/non-operated neuroforamens in each and whole lumbar level (L2–S1) were as follows: L2–L3, 1.93/0.27; L3–L4, 2.93/0.33; L4–L5, 2.83/0.99; L5–S1, 2.71/1.20; L2–S1, 2.72/1.05. Comparison of the mean MRI grade between operated and non-operated neuroforamens was significantly different, and at all levels (all p<0.001) (Table 2).
Interobserver agreement in the grading of foraminal stenosis was very good in all operated neuroforamens, except for at the L5–S1 level (κ value: L2–L3, 0.848; L3–L4, 1.000; L4–L5, 0.805; L5–S1, 0.313), and more than moderate in non-operated neuroforamens at all lumbar levels (κ value: L2–L3, 0.545; L3–L4, 0.615; L4–L5, 0.705; L5–S1, 0.674). Intraobserver agreement one month later by researcher 2 was more than good in operated neuroforamens (κ value: L2–L3, 1.000; L3–L4, 1.000; L4–L5, 0.729; L5–S1, 0.715) and more than moderate in non-operated neuroforamens (κ value: L2–L3, 1.000; L3–L4, 0.545; L4–L5, 0.725; L5–S1, 0.877) (Table 3).
MRI is a very useful tool in the evaluation of lumbar foraminal stenosis. Nonetheless, there has been relatively few reports on the reliability of MRI-based grading or classification of lumbar foraminal stenosis, and the ability to predict surgical outcomes. Grading systems based on the degree of epidural fat obliteration12), according to locations of epidural fat obliteration in four quadrants of intervertebral foramen1), and, more recently, based on the type of stenosis, amount of fat obliteration, and presence of nerve root compression9) have been proposed. However, investigations into these MRI grading systems have simply focused on evaluation of lumbar foraminal stenosis, and have not made any clinical correlations, such as operative indication in relation to MRI grade. We wished to investigate how reliable and how useful MRI grading system can be clinically as applying MRI grading system to patients with lumbar foraminal stenosis who had to be performed surgery.
In this study, operated neuroforamens were compared with non-operated, asymptomatic neuroforamens using a common MRI grading system9). All patients underwent surgery, because the pain resulting from lumbar foraminal stenosis did not subside despite enough conservative treatment. The mean MRI grade of operated neuroforamens at L3–L4, L4–L5, and L5–S1 was more than 2.5, though this was not the case for the L2–L3 level. MRI grade of operated neuroforamens was significantly greater different to non-operated neuroforamens (p<0.001). Thus, if MRI grade is Grade 2 or Grade 3 in patients diagnosed with lumbar foraminal stenosis, decompressive surgery can be considered as an optimal treatment over long-term conservative treatment.
We found that interobserver agreement for operated neuroforamens at the L5–S1 level (κ=0.313, fair) was relatively lower than for levels L2–L5 (κ=0.804, good). There are several reasons why this might be the case. First, pedicle width is widest at L5 in the lumbar spine13); about two key sagittal images to evaluate foraminal stenosis can be obtained at the L5–S1 level, while only one key image can be obtained at other levels. Thus, in cases that have symptomatic foraminal stenosis at the L5–S1 level, a key image without stenosis can be evaluated although there is a key image with stenosis. Second, the lower lumbar nerve roots, especially the L5 nerve root, are characterized by a more oblique course throughout the lateral canal6). In some cases, the sagittal MR image of the L5 nerve root can be cut obliquely, unlike the image being cut in the axis line of the nerve, which as a result can be hard to correctly evaluate. Third, the two researchers in our study were a radiologist and neurosurgeon. It is possible that the neurosurgeon evaluated foraminal stenosis with more consideration to the extraforaminal component, such as the sacral alar and L5 transverse process, which can compress the nerve root as well as intraforaminal component. Neurosurgeons have observed directly in operative field that the L5 nerve root is compressed by an extraforaminal component in L5–S1 foraminal stenosis, which has more causative factors than other levels.
Our data indicated that the MRI grading system output correlated with clinical aspects such as pain intensity and radiologic findings, which was useful to evaluate the causes and severity of a lesion, and was helpful to make a surgical decision. However, because reliability of the MRI grading system at L5–S1 level was significantly lower than at other levels, careful judgement is needed. Therefore, various clinical factors should be considered in making treatment plans, especially at L5–S1 level.
There are several limitations to our study. One limitation is that a precise κ value at upper lumbar levels (L1–L4) could not be obtained because of the small sample size of lumbar foraminal stenosis at the upper lumbar level. A second limitation is that the follow-up MRI analysis by researcher 2 (to test intraobserver agreement) was only one month later. Such a short follow-up period could have biased MRI analysis because the researcher may have remembered MR images. A third limitation is that no comparison with conservative treatment group was made. More conclusive evidence for whether the MRI grading system is useful as a diagnostic tool for surgery could be gained by comparing the MRI grade of patients who underwent surgery with those who were treated conservatively.
The MRI grading system is a useful tool that allows lumbar foraminal stenosis to be evaluated more objectively. For Grade 3, surgical treatment can be considered over conservative treatment. However, the MRI grading system alone is less reliable for symptomatic, L5–S1 foraminal stenosis, as indicated by a lower margin of agreement in operated neuroforamens than in other levels. Therefore, various clinical factors as well as an MRI grading system are required for surgical decision-making.
References
1. Attias N, Hayman A, Hipp JA, Noble P, Esses SI. Assessment of magnetic resonance imaging in the diagnosis of lumbar spine foraminal stenosis--a surgeon’s perspective. J Spinal Disord Tech. 19:249–256. 2006.

2. Chang SB, Lee SH, Ahn Y, Kim JM. Risk factor for unsatisfactory outcome after lumbar foraminal and far lateral microdecompression. Spine (Phila Pa 1976). 31:1163–1167. 2006.

3. Fujiwara A, An HS, Lim TH, Haughton VM. Morphologic changes in the lumbar intervertebral foramen due to flexion-extension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine (Phila Pa 1976). 26:876–882. 2001.

4. Grenier N, Kressel HY, Schiebler ML, Grossman RI, Dalinka MK. Normal and degenerative posterior spinal structures: MR imaging. Radiology. 165:517–525. 1987.

5. Hasegawa T, An HS, Haughton VM, Nowicki BH. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryo-microtome study in cadavera. J Bone Joint Surg Am. 77:32–38. 1995.
6. Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976). 25:389–394. 2000.
7. Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine (Phila Pa 1976). 16:1312–1320. 1991.

8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 33:159–174. 1977.

9. Lee S, Lee JW, Yeom JS, Kim KJ, Kim HJ, Chung SK, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 194:1095–1098. 2010.

10. Porter R, Hibbert C, Evans C. The natural history of root entrapment syndrome. Spine (Phila Pa 1976). 9:418–421. 1984.

11. Shim YJ, Ha HG, Lee JS, Kim YS, Park MS, Kim JS. Microsurgical decompression for lumbar stenosis via unilateral laminotomy. J Korean Neurosurg Soc. 29:1505–1513. 2000.
Fig. 1
Four-point-scale for grading of lumbar foraminal stenosis in sagittal MRI suggested by Lee et al.9). A: Grade 0 (normal). Sagittal cross section through foramen shows normal neuroforamen. B: Grade 1 (mild foraminal stenosis). Sagittal cross section through foramen shows perineural fat obliteration in two opposing directions (vertical or transverse, arrows). C: Grade 2 (moderate foraminal stenosis). Sagittal cross section through foramen shows perineural fat obliteration in four directions (arrows). D: Grade 3 (severe foraminal stenosis). Sagittal cross section through foramen shows morphologic changes in the nerve root. MRI: magnetic resonance imaging.
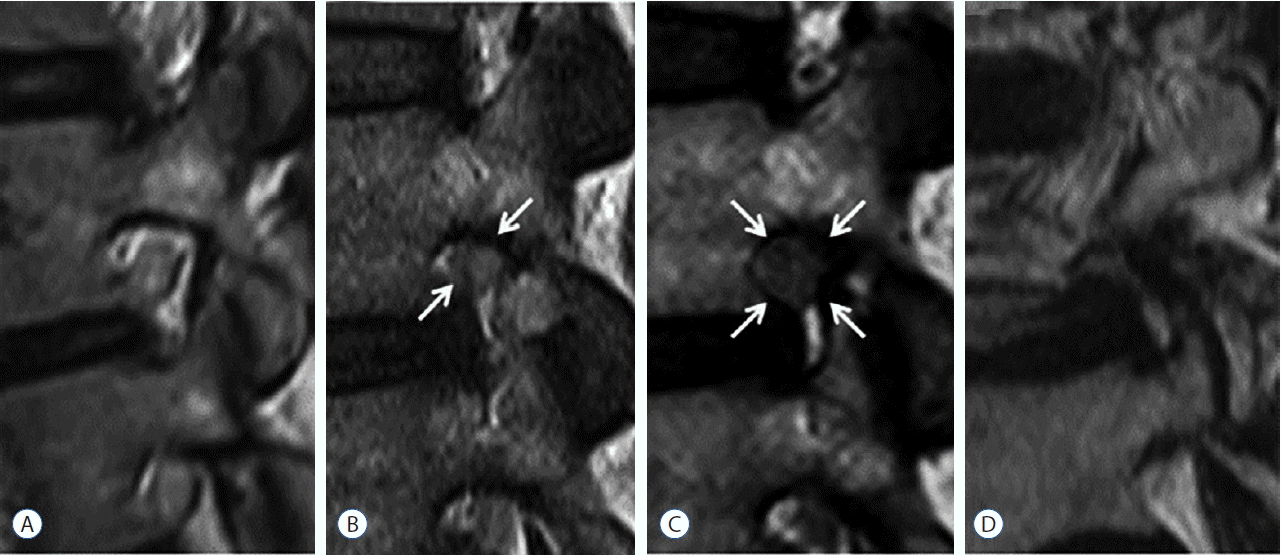
Fig. 2
T1-weighted sagittal image of a 44-year-old man with left lower extremity pain. A: Sagittal MR image shows Grade 3 foraminal stenosis at left foramen of the L3–L4 level. B: Sagittal MR image shows Grade 0 of normal neuroforamen at right foramen of the L3–L4 level. MR: magnetic resonance.
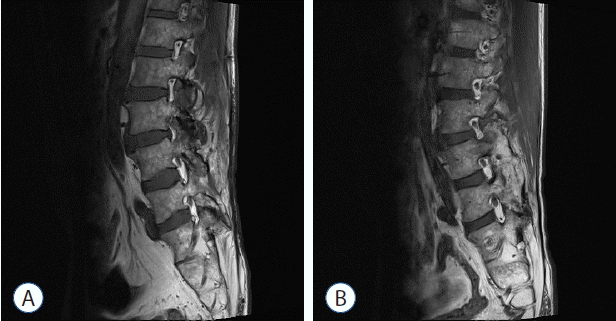
Table 1
Demographic characteristics of patients with lumbar foraminal stenosis who underwent surgery
| Characteristics | Value |
|---|---|
| Sex | |
| Female | 54 (54.5) |
| Male | 45 (45.5) |
|
|
|
| Age (yrs) | 62.6±11.6 |
|
|
|
| Mean symptom duration | 8.6 weeks |
|
|
|
| VAS | |
| Preoperative | 7.7±1.5 |
| Postoperative | 1.6±0.9 |
Table 2
The number of analyzed neuroforamens and mean MRI grade at each level of the lumbar spine
Table 3
Interobserver and intraobserver agreement analyzed using κ statistics




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download