INTRODUCTION
MATERIALS AND METHODS
Animals
Experimental design and surgery
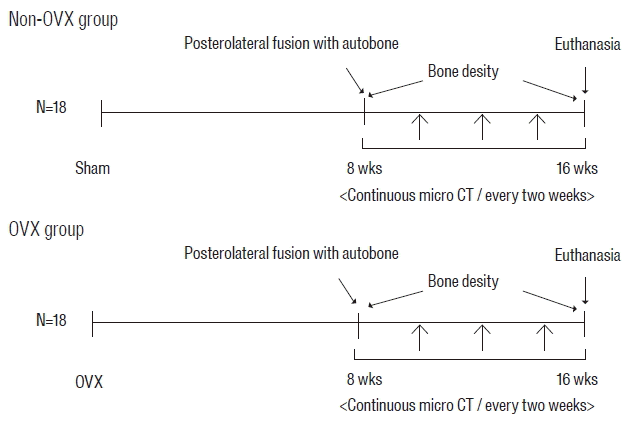 | Fig. 1Experimental groups and time schedule. Eighteen rats underwent bilateral OVX (OVX group), and another 18 rats underwent a sham operation (non-OVX group). Eight weeks after OVX, all rats underwent unilateral spinal fusion using autologous iliac bone. Sequential micro-CT evaluations in all rats were obtained every 2 weeks for 8 weeks after surgery. Bone density of all rats was assessed at 2 days and 8 weeks after fusion surgery. OVX: ovariectomy, micro-CT: microcomputed tomography. |
 | Fig. 2Assessment of fusion processes using three-dimensional (3D) images. The scheme and 3D reconstructed images show nonfusion (A) and fusion (B). Fusion was defined as the simultaneous presence of bridging bone and the connection of bridging bone to both upper and lower TPs. The shape of the bone fragments in 3D reconstructed image A (asterisk) does not appear as compact as that in 3D reconstructed image B (asterisk). VB: vertebral body, TP: transverse process. |
Statistical analysis
RESULTS
Bone density
Fusion rate and fusion process
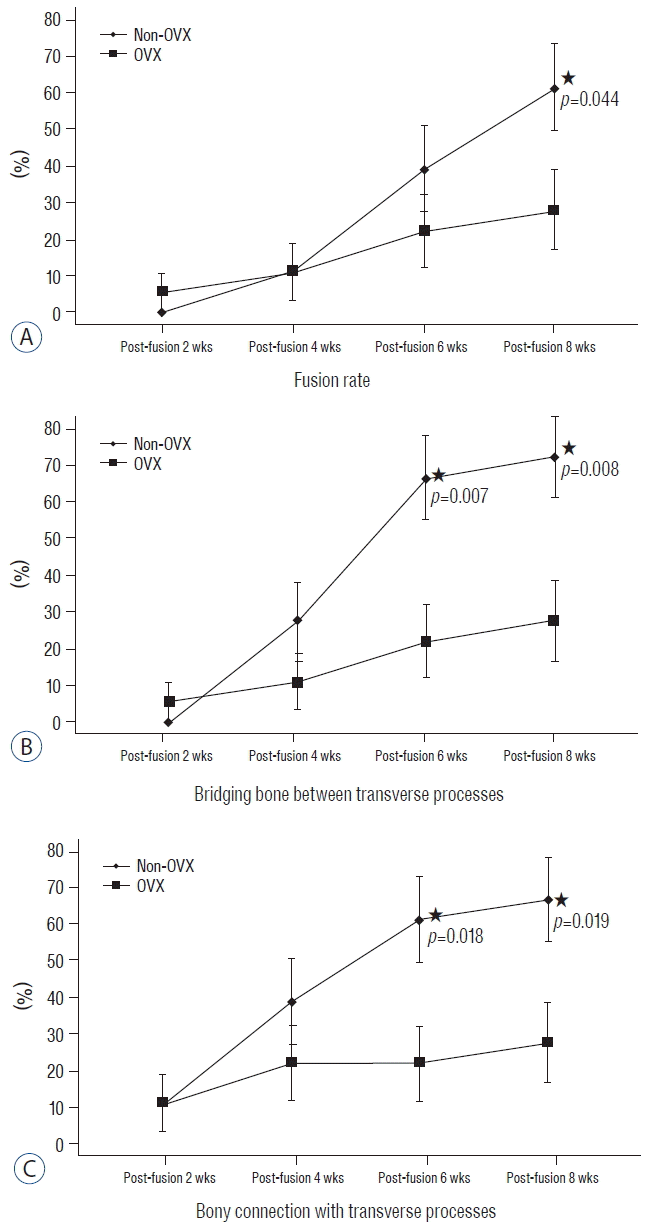 | Fig. 3Comparison of the fusion process between two groups. This graph shows the difference between non-OVX and OVX groups in fusion rate (A), and the ratios of the presence of a bony connection with the TP (B) and bridging bone between TPs (C) at postoperative weeks 2, 4, 6 and 8 (asterisk, p<0.05). OVX: ovariectomy, TP: transverse process. |
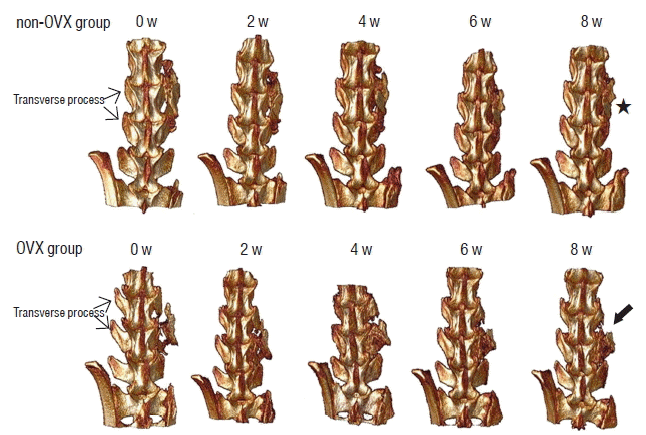 | Fig. 4Micro-CT scanning of fusion masses. The figures show 3D reconstructed images from both groups after surgery. The picture in the upper row shows serial 3D reconstructed images from a rat in the non-OVX group. The grafted bone materials were inserted on the fusion bed between the L4 and L5 TPs. The shape of the bridging bone 8 weeks after surgery (asterisk) was more compact than that in the early period after surgery. The picture in the lower row is a 3D reconstructed image from a rat in the OVX group. The shape of the grafted bone at 8 weeks was less compact in the OVX group (arrow) than in the non-OVX group. Micro-CT: microcomputed tomography, OVX: ovariectomy, TP: transverse process. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download