Abstract
Objective
The purpose of this study is to evaluate the associations between 30-day mortality and various radiological and clinical factors in patients with traumatic acute subdural hematoma (SDH). During the 11-year study period, young patients who underwent surgery for SDH were followed for 30 days. Patients who died due to other medical comorbidities or other organ problems were not included in the study population.
Methods
From January 1, 2004 to December 31, 2014, 318 consecutive surgically-treated traumatic acute SDH patients were registered for the study. The Kaplan–Meier method was used to analyze 30-day survival rates. We also estimated the hazard ratios of various variables in order to identify the independent predictors of 30-day mortality.
Results
We observed a negative correlation between 30-day mortality and Glasgow coma scale score (per 1-point score increase) (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.52–0.70; p<0.001). In addition, use of antithrombotics (HR, 2.34; 95% CI, 1.27–4.33; p=0.008), history of diabetes mellitus (HR, 2.28; 95% CI, 1.20–4.32; p=0.015), and accompanying traumatic subarachnoid hemorrhage (hazard ratio, 2.13; 95% CI, 1.27–3.58; p=0.005) were positively associated with 30-day mortality.
Conclusion
We found significant associations between short-term mortality after surgery for traumatic acute SDH and lower Glasgow Coma Scale scores, use of antithrombotics, history of diabetes mellitus, and accompanying traumatic subarachnoid hemorrhage at admission. We expect these findings to be helpful for selecting patients for surgical treatment of traumatic acute SDH, and for making accurate prognoses.
Traumatic acute subdural hematoma (SDH) is a common condition confronting neurosurgeons, and it is reported that the percentage of acute SDH in patients admitted with a traumatic brain injury (TBI) is approximately 10–20%16,24). A previous study reported that approximately 60% of severe TBI patients have acute SDH to various extents. We evaluated the 30-day mortality rate following surgery for traumatic acute SDH because identification of individual risk factors for early death is important for informing clinical management14). According to recent studies, the 30-day mortality rate after surgery for TBI in approximately 17–26%14,24). One study including mostly elderly patients (average age 70.9 years, range 14.1 years) with various medical comorbidities found that the 30-day mortality rate after surgery for acute SDH was about 2-fold higher in patients over 60 years old24). The authors suggested that death following SDH is influenced both by the extent of neurological damage and by the overall health of the patient at the time of surgery24). Therefore, the extent of the pure effects of severe TBI on short-term mortality is ambiguous because of the short-term mortality associated with various radiological and clinical factors.
We here evaluate the associations between 30-day mortality and various radiological and clinical factors in young patients with no deaths due to medical comorbidities or other organ problems. Patients were followed during the initial 30 postoperative days after surgery for traumatic acute SDH.
We retrospectively collected traumatic acute SDH patient data from our two hospitals from January 1, 2004 to December 31, 2014. Since 2000, the medical center has been obligated to record all TBI patients in the registry. After retrieving data from patients with traumatic acute SDH from the registry, all medical records including operative records were reviewed by two specialized research teams using an Electronic Medical Records system database. Non-surgical patients and patients with surgery performed more than 48 hours after head trauma were excluded. We also excluded patients younger than 15 years and those older than 65 years due to increased risk of death from various medical comorbidities or other organ problems, rather than neurological conditions. A series of 318 patients was identified for the study (Fig. 1).
All operations were performed by four senior neurosurgeons. Surgery was performed in a standardized manner using a trauma flap. For patients taking warfarin, we routinely administered intravenous vitamin K. Patients were further managed in the intensive care unit according to a standard protocol, and the target glucose concentration range was 80 to 110 mg/dL with insulin therapy. In most cases, decompressive craniectomy and hematoma evacuation with duraplasty were performed. A decompressive craniectomy was performed based on the surgeon’s preference and brain edema in the operative field. The decision to perform this craniectomy was not strictly standardized due to the retrospective nature of the study. There was no significant difference between the alive and dead patient groups based on operation type (craniectomy and craniotomy) (Table 1).
Re-operation (bilateral hemicraniectomy, hematoma evacuation or drainage, or bifrontal craniectomy) was conducted in 31 patients: new lesions appeared in the contralateral portion (SDH or epidural hematoma [EDH]) in 11 patients, and aggravation of initial lesions or bleeding complications (SDH, traumatic intracerebral hematoma [TICH], exacerbation of intraventricular hemorrhage [IVH] or EDH, or rebleeding) occurred in 20 patients, as assessed by immediate postoperative computed tomography (CT) scans.
This study was approved by the Institutional Review Boards of our hospitals in both Seoul and Guri.
Initial CT scans of all 318 traumatic acute SDH patients were assessed by two senior neurosurgeons. Mean radiologic measurements (i.e., midline shift, TICH volume) were calculated and used in the analysis. We evaluated the bilaterality of the SDH and measured the midline shift in millimeters, which was compared with a line drawn between the anterior and posterior attachments of the falx to the inner table of the skull. We also evaluated the accompanying TBI in all 318 patients. We defined TICH lesions as those with a solid and well-defined appearance and fewer well-defined areas of mixed attenuation5). In well-defined TICH lesions, we measured the baseline volume of the ICH using the formula (A×B×C)/2, where A is the greatest hemorrhage diameter in the CT scan, B is the diameter measured 90 degrees to A, and C is the approximate number of 10-mm CT slices showing hemorrhage19). The diagnosis of traumatic subarachnoid hemorrhage (tSAH) was based on the presence of blood in the subarachnoid space in the admission CT scans and was classified according to the Fisher grade8). In all cases where the sequence of SAH and trauma was ambiguous, we routinely evaluated for vascular aneurysm through CT angiogram before surgery. In addition, we evaluated for other potential brain injuries including IVH, EDH, and skull fracture.
We reviewed the medical charts and laboratory results for all 318 patients who met the inclusion criteria. We investigated age, gender, mechanism of trauma, and Glasgow coma scale (GCS) scores on admission. Accompanying injuries other than brain injuries were also evaluated. These non-lethal accompanying injuries were not associated with 30-day mortality in any patients in the study.
Hypertension was defined as the previous use of antihypertensive medication, medical records indicating a history of hypertension, or a confirmed history of hypertension. A history of diabetes was defined as previous use of antidiabetic oral medications, including the use of intravenous insulin therapy, medical records indicating a history of diabetes, or a confirmed history of diabetes. We classified stress-induced hyperglycemia (SIH) as random blood glucose >200 mg/dL on admission without a history of diabetic medication or diagnosis of diabetes. In addition, we investigated other major comorbidities that might affect the 30-day mortality after surgery. A history of smoking was defined to include former and current smokers, and a history of drinking was defined to include former and current drinkers. We also investigated the use of antithrombotic medications in all patients, including anticoagulants (warfarin) and antiplatelet agents (aspirin and clopidogrel). No patients were taking both anticoagulants and antiplatelet agents.
In-hospital 30-day mortality was the main outcome variable of this analysis to evaluate the effect of the accompanying brain injury and patient clinical factors on mortality of traumatic acute SDH in patients between 15 and 65 years of age. Deaths due to other medical conditions or other organ problems after surgery were not considered in this study.
The baseline characteristics of patient data are presented as mean±standard deviation and number/percentage. The Chi-square test for dichotomous variables and Student’s t-test for continuous variables were used to assess clinical differences between patients who survived and those who died within 30 days of surgery.
Univariate associations between radiologic findings, patient clinical factors, and 30-day mortality were assessed using Kaplan–Meier survival analysis, and significance was determined using the log-rank test. A p-value less than 0.05 was considered statistically significant.
We first calculated hazard ratios (HRs) with 95% confidence intervals (CIs) for 30-day mortality based on the various radiologic findings and patient clinical factors using univariate Cox proportional hazards regression analyses. Covariates for the multivariate Cox model were selected on the basis of a p-value <0.05 in a univariate analysis, in addition to age and gender. We then estimated HRs with 95% CIs using a multivariate Cox proportional hazards regression model in order to identify potential predictors of 30-day mortality. We evaluated multicollinearity for all variables with a multivariate Cox proportional hazards regression model. We calculated variance inflation factor (VIF), which is the inverse of 1–R2 and shows how much the variance of the coefficient estimate is inflated by multicollinearity in the model23). We also evaluated log minus log plots for all variables in order to test the validity of the proportionality of hazards assumption over time, and all variables met this assumption.
Statistical analyses were performed in R version 3.1.2 and SPSS for Windows, version 22.0 (IBM Inc., Chicago, IL, USA).
We included 318 consecutive patients between 15 and 65 years of age who underwent surgery for traumatic acute SDH between January 1, 2004 and December 31, 2014. The average age of the patients was 47.8 years, and 75.5% were men. There were significant differences in Glasgow coma scale (GCS) score, accompanying TICH and tSAH together or tSAH alone, midline shift, diabetes, and SIH between patients who survived and patients who had died within 30 days. Further descriptive data are shown in Table 1.
We investigated accompanying injuries other than brain injuries, and these were not associated with 30-day mortality in patients with traumatic acute SDH in this study (Table 2).
Fig. 2 shows the initial distribution of accompanying brain injuries in patients who were alive or dead within 30 days of surgery for acute SDH. We found significantly higher 30-day mortality rates in patients with accompanying TICH and tSAH together or tSAH alone.
Fig. 3 and 4 present the Kaplan–Meier curve with the log-rank test showing survival probability within 30 days after surgery based on initial accompanying brain injury and clinical and radiologic factors. The overall 30-day survival probability reached a plateau near 80%. There were associations between 30-day mortality and GCS score, TICH and tSAH together or tSAH alone, midline shift >5 mm, diabetes, SIH, and anticoagulant use. There was a tendency towards a higher mortality rate in the tSAH group and antithrombotics-use group immediately after surgery. However, mortality rates increased around 6 days after surgery in the SIH group and nine days after surgery in the diabetes group, and then decreased with time thereafter.
Table 3 shows the percentage of survivors at the end point (30 days after surgery) in the Kaplan–Meier test and the univariate HRs of clinical and radiologic variables associated with 30-day mortality after surgery. In the univariate analyses, GCS score (per 1 point score increase) was negatively associated with 30-day mortality, while tSAH presence, midline shift (per 1 mm increase), diabetes, SIH, and antithrombotic use were positively associated with 30-day mortality. There was no association between 30-day mortality after surgery and gender, age, TICH presence, bilateral acute SDH, smoking, or antiplatelet agent use. We evaluated multicollinearity of the variables before performing a multivariate Cox regression analysis. The VIF was 1.014 for antithrombotics, 1.197 for tSAH, 1.098 for diabetes, 1.372 for GCS score, 1.355 for midline shift, 1.103 for SIH, 1.236 for age, and 1.040 for gender. In the multivariate Cox regression analyses, we observed that GCS score (HR, 0.60 ; 95% CI, 0.52–0.70 ; p<0.001; per 1 point score increase) was inversely associated with 30-day mortality after surgery for acute SDH. However, antithrombotic use (HR, 2.34 ; 95% CI, 1.27–4.33 ; p=0.007), diabetes (HR, 2.28 ; 95% CI, 1.20–4.32 ; p=0.012) and tSAH presence (HR, 2.13 ; 95% CI, 1.27–3.58 ; p=0.004) showed independent associations with short-term mortality in surgically-treated acute SDH patients (Fig. 5). Although SIH showed about a 1.8-fold increase in 30-day mortality in the univariate analysis (HR, 1.75 ; 95% CI, 1.03–2.98 ; p=0.037), this factor fell short of achieving statistical significance in the multivariate analysis (HR, 1.55 ; 95% CI, 0.86–2.78 ; p=0.145).
Our study shows that a decreased GCS score, accompanying tSAH, history of diabetes, and antithrombotic use are independent predictors of 30-day mortality after surgery for traumatic acute SDH in patients between 15 and 65 years of age. This finding excludes patients whose deaths were related to other medical conditions or organ problems. We observed that there were no associations between 30-day mortality and patient age, gender, or various accompanying brain injuries other than tSAH. In addition, midline shift and SIH also showed no significant correlation with 30-day mortality in the multivariate analysis. In the antithrombotic-use group, the anticoagulant-agent group showed a significant correlation with 30-day mortality in the univariate analysis, whereas the antiplatelet group did not. However, we did not divide the antithrombotic group into an anticoagulant group and an antiplatelet group for the multivariate analysis because the resultant small numbers would have decreased the statistical power (Fig. 5).
Sixty-nine patients died within 30 days after surgery for traumatic acute SDH, a mortality rate of 21.7%. Recently, Huang et al. reported a 30-day mortality rate of 26.4% in traumatic brain-injury patients undergoing decompressive craniectomy14). Our study has a mortality rate about five percentage points lower, and this difference might be explained by the fact that patients older than 65 years, who are more vulnerable to death after major trauma, were excluded from our study13). In addition, we excluded patients who died from other medical conditions or other non-neurological organ problems. Conversely, a recent U.S. study describes a 30-day mortality rate of 17% in surgically-treated SDH patients24). However, the researchers randomly selected surgical patients from a large database and focused mainly on the comorbidities in elderly patients. Therefore, the severity of the traumatic SDH and the presence of accompanying TBI are ambiguous in that study. The small number of patients in our study might also be a potential cause of the difference in mortality rate.
In our study, a lower GCS score was associated with the 30-day mortality rate. Many previous studies have reported a correlation between low GCS scores and a poor outcome/short-term mortality after TBI1,14,26,27). We confirmed that GCS score can be a prognostic predictor of short-term mortality in patients who undergo decompressive craniectomy.
The use of antithrombotic medication was an independent predictor of 30-day mortality. Several previous studies have shown the adverse effects of anticoagulant use on prognosis and mortality in TBI3,6,9,11,27). A U.S. study reported that anticoagulation with warfarin does not adversely impact mortality or length-of-stay outcomes in patients with head injuries30). The researchers stated that the higher mortality in anticoagulated elderly trauma patients was more attributable to the effects of age and comorbidities than the effects of warfarin exposure. Devereaux et al. reported that administration of aspirin before surgery and throughout the early postsurgical period had no significant effect on the rate of composite death7).
In the univariate analysis in our study, diabetes and SIH were associated with 30-day mortality. However, only diabetes was an independent predictor of 30-day mortality in the multivariate analysis. Several studies have shown an association between mortality or neurological outcome and hyperglycemia after TBI12,15,22,29). However, these studies included all hyperglycemic patients without considering the patients’ history of diabetes. Bosarge et al. found that SIH was associated with higher mortality after severe TBI than diabetic hyperglycemia4). However, diabetic hyperglycemic patients might have some degree of stress response invoking their hyperglycemia. In our study, we included all patients with diagnosed diabetes including those with hyperglycemia and normoglycemia. Two previous studies have reported that diabetes was associated with mortality in TBI20,25). Ley et al. proposed that TBI inflammatory cytokines might lead to disruption in the display of the glucose transporter on the cell surface, while simultaneously causing inappropriate activation of the glucose synthase system20). Consequently, glucose availability from a relative insulin deficiency that is amplified in diabetes mellitus leads to increased mortality because of the inability to meet cellular energy demands. In addition, TBI might cause cerebral vascular injury, which leads to ischemic lesions in the brain17). According to a recent study, ischemic stroke is the second most common cause of death for patients with TBI, after decompressive craniectomy14,21). Khoury et al. reported that diabetic patients showed a 3- to 4-fold increased incidence rate of ischemic stroke compared with those without diabetes mellitus18).
Lastly, accompanying tSAH was also an independent predictor of 30-day mortality. Several studies have reported that tSAH was an independent predictor of TBI mortality and severe neurological outcome2,10,21,28,31). Aminmansour et al. described an association between tSAH and an increased incidence of cerebral vasospasm, with higher probability and greater severity in patients with severe TBI2). In addition, Zubkov et al. reported an increase in post-traumatic vasospasm in patients with EDH, SDH, and ICH32). Therefore, tSAH in our study might have some additional effects on the incidence of vasospasm, relative to the effect of an isolated tSAH lesion, because our study was based on patients with traumatic SDH. In addition, a recent study from China showed that tSAH was an independent predictor of post-traumatic cerebral infarction21).
Although midline shift was associated with 30-day mortality in the univariate analysis, it fell short of significance in the multivariate analysis. Aarabi et al. reported that decompressive craniectomy was associated with a better-than-expected functional outcome in patients with a malignant swelling from to severe head injuries1). In our study, most patients underwent decompressive craniectomies, which might explain the lack of a significant relationship between midline shift and short-term mortality in the multivariate analysis. A recent study has also reported that midline shift was not associated with 30-day mortality in patients undergoing decompressive craniectomy in a multivariate analysis14).
There are some limitations to the present study. Because of the retrospective nature of this study, there were inconsistencies in the duration between the immediate postoperative CT scan and the follow-up CT scan. Therefore, we could not accurately evaluate the cause of death. In addition, there was the possibility of incorrect or missing patient data. Therefore, the present study might be less accurate than prospectively planned research. Furthermore, we could not obtain information regarding HbA1c on admission, because this value is not routinely measured in the emergency department. Therefore, we might have underestimated the number of diabetic patients. The present study was performed in only two hospitals, so the generalizability of these findings might be limited. Although this study was performed in two major urban hospitals over an 11-year period, the small number of patients might limit the statistical power. The small number of patients with antithrombotics used in our study, due to the study characteristics (less than 65 years old), means that we could not clearly evaluate the influence of antithrombotic agents on the short-term mortality of acute SDH in relatively younger patients of our study, compared to the previous studies. However, because of our exclusion criteria, this study did include a relatively large number of patients compared with previous studies.
Regardless of the limitations described above, the present study suggests several predictors that deserve consideration when considering short-term mortality after surgery for traumatic acute SDH in patients less than 65 years old. We found a significant association between short-term mortality and lower GCS score, antithrombotic use, history of diabetes mellitus, and accompanying tSAH at admission in surgically-treated, traumatic acute SDH patients between 15 and 65 years of age. The independent factors associated with short-term mortality after TBI reported in previous studies that included the elderly were also similar with short-term mortality after surgery for traumatic acute SDH in patients less than 65 years old. We expect these findings to be helpful for selecting patients for surgical treatment of traumatic acute SDH, and for making accurate prognoses. Additional prospective multi-center studies will be required to further evaluate these findings.
References
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 104:469–479. 2006.

2. Aminmansour B, Ghorbani A, Sharifi D, Shemshaki H, Ahmadi A. Cerebral vasospasm following traumatic subarachnoid hemorrhage. J Res Med Sci. 14:343–348. 2009.
3. Beynon C, Potzy A, Sakowitz OW, Unterberg AW. Rivaroxaban and intracranial haemorrhage after mild traumatic brain injury: A dangerous combination? Clin Neurol Neurosurg. 136:73–78. 2015.

4. Bosarge PL, Shoultz TH, Griffin RL, Kerby JD. Stress-induced hyperglycemia is associated with higher mortality in severe traumatic brain injury. J Trauma Acute Care Surg. 79:289–294. 2015.

5. Cepeda S, Gómez PA, Castaño-Leon AM, Martínez-Pérez R, Munarriz PM, Lagares A. Traumatic intracerebral hemorrhage: risk factors associated with progression. J Neurotrauma. 32:1246–1253. 2015.

6. Collins CE, Witkowski ER, Flahive JM, Anderson FA Jr, Santry HP. Effect of preinjury warfarin use on outcomes after head trauma in Medicare beneficiaries. Am J Surg. 208:544–549.e1. 2014.

7. Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 370:1494–1503. 2014.
8. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 6:1–9. 1980.

9. Franko J, Kish KJ, O’Connell BG, Subramanian S, Yuschak JV. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 61:107–110. 2006.

10. Gómez PA, de-la-Cruz J, Lora D, Jiménez-Roldán L, Rodríguez-Boto G, Sarabia R, et al. Validation of a prognostic score for early mortality in severe head injury cases. J Neurosurg. 121:1314–1322. 2014.

11. Grandhi R, Harrison G, Voronovich Z, Bauer J, Chen SH, Nicholas D, et al. Preinjury warfarin, but not antiplatelet medications, increases mortality in elderly traumatic brain injury patients. J Trauma Acute Care Surg. 78:614–621. 2015.

12. Griesdale DE, Tremblay MH, McEwen J, Chittock DR. Glucose control and mortality in patients with severe traumatic brain injury. Neurocrit Care. 11:311–316. 2009.

13. Howard MA 3rd, Gross AS, Dacey RG Jr, Winn HR. Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg. 71:858–863. 1989.

14. Huang YH, Lee TC, Lee TH, Liao CC, Sheehan J, Kwan AL. Thirty-day mortality in traumatically brain-injured patients undergoing decompressive craniectomy. J Neurosurg. 118:1329–1335. 2013.

15. Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 58:47–50. 2005.

16. Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo). 54:887–894. 2014.

17. Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, et al. Cerebral vascular injury in traumatic brain injury. Exp Neurol 275 Pt. 3:353–366. 2016.

18. Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke J Cereb Circ. 44:1500–1504. 2013.
19. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 27:1304–1305. 1996.

20. Ley EJ, Srour MK, Clond MA, Barnajian M, Tillou A, Mirocha J, et al. Diabetic patients with traumatic brain injury: insulin deficiency is associated with increased mortality. J Trauma. 70:1141–1144. 2011.

21. Liu S, Wan X, Wang S, Huang L, Zhu M, Zhang S, et al. Posttraumatic cerebral infarction in severe traumatic brain injury: characteristics, risk factors and potential mechanisms. Acta Neurochir (Wien). 157:1697–1704. 2015.

22. Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 11:151–157. 2009.

23. Logistic Regression Using SAS®: Theory and Application, Second Edition. Available: https://www.sas.com/store/books/categories/usage-and-reference/logistic-regression-using-sas-theory-and-application-second-edition/prodBK_61340_en.html. Accessed 14 October 2015.
24. Lukasiewicz AM, Grant RA, Basques BA, Webb ML, Samuel AM, Grauer JN. Patient factors associated with 30-day morbidity, mortality, and length of stay after surgery for subdural hematoma: a study of the American College of Surgeons National Surgical Quality Improvement Program. J Neurosurg. 124:1–7. 2015.

25. Lustenberger T, Talving P, Lam L, Inaba K, Bass M, Plurad D, et al. Effect of diabetes mellitus on outcome in patients with traumatic brain injury: a national trauma databank analysis. Brain Inj. 27:281–285. 2013.

26. MRC CRASH Trial Collaborators. Perel P, Arango M, Clayton T, Edwards P, Komolafe E, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 336:425–429. 2008.

27. Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 24:329–337. 2007.

28. Parchani A, El-Menyar A, Al-Thani H, El-Faramawy A, Zarour A, Asim M, et al. Traumatic subarachnoid hemorrhage due to motor vehicle crash versus fall from height: a 4-year epidemiologic study. World Neurosurg. 82:e639–e644. 2014.

29. Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 46:335–342. discussion 342–343. 2000.

30. Wojcik R, Cipolle MD, Seislove E, Wasser TE, Pasquale MD. Preinjury warfarin does not impact outcome in trauma patients. J Trauma. 51:1147–1151. discussion 1151–1152. 2001.

Fig. 1
Flow chart of the process for selecting eligible patients from our hospital’s Traumatic Brain Injury registry during the period from January 1, 2004 to December 31, 2014.

Fig. 2
Dichotomized analysis of the distribution of initial accompanying brain injuries in patients who survived or died within 30 days of surgery for acute subdural hematoma. TICH: traumatic intracerebral hematoma, tSAH: traumatic subarachnoid hemorrhage, IVH: intraventricular blood, EDH: epidural hematoma. * p<0.05.
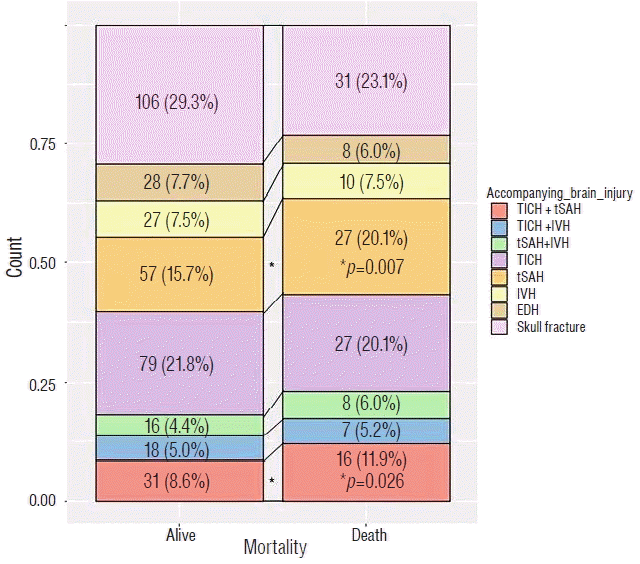
Fig. 3
Kaplan–Meier curve with log-rank test showing survival probability within 30 days of surgery, based on initial accompanying brain injury. CI: conÿdence interval, TICH: traumatic intracerebral hematoma, tSAH: traumatic subarachnoid hemorrhage, IVH: intraventricular blood, EDH: epidural hematoma.

Fig. 4
Kaplan–Meier curve with log-rank test showing survival probability within 30 days of surgery based on clinical and radiologic factors. GCS: Glasgow coma scale, SDH: subdural hematoma, SIH: stress-induced hyperglycemia.

Fig. 5
Multivariate hazard ratios of clinical and radiologic factors associated with 30-day mortality after surgery. HR: hazard ratio, CI: confidence interval, tSAH: traumatic subarachnoid hemorrhage, SIH: stress-induced hyperglycemia, GCS: Glasgow coma scale. *p<0.01, †p<0.05, ‡p<0.001.
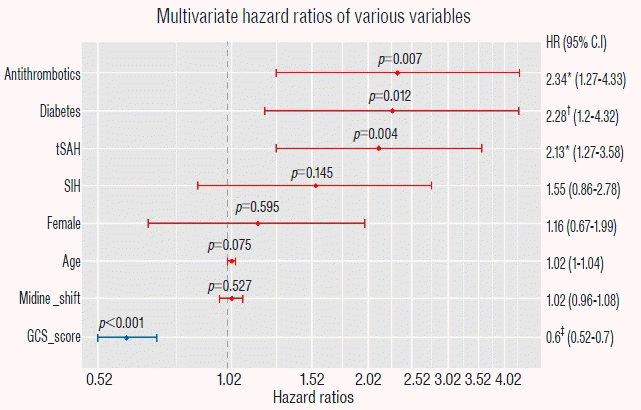
Table 1
Clinical characteristics and radiologic ÿndings of surgically-treated acute subdural hematoma patients, within 30 days after surgery
Table 2
Accompanying injuries other than brain injuries in patients in the study
Table 3
Kaplan–Meier and univariate hazard ratios for clinical and radiologic factors associated with 30-day mortality after surgery




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download