INTRODUCTION
Fusion operations are widely performed to treat lumbar spinal stenosis. The instrumentation provides stability for the first few months, and bone union, also called solid fusion, maintains stability thereafter11,29). It is widely accepted that an autologous bone graft using an iliac bone harvest is the gold standard in spinal fusion because it can provide all three essential properties (osteogenesis, osteoinduction, and osteoconduction) required for bone fusion6,8,18). However, the major disadvantage of this procedure is the unavoidable donor site morbidity8,18). Moreover, more recent minimally invasive surgeries, such as direct lumbar interbody fusion (DLIF), make it difficult to obtain adequate amounts of local autologous bone chips.
There have been many efforts to enhance fusion rate, with the hope of eventually entirely replacing the autologous bone grafts21). One such development is a bone graft substitute, such as demineralized bone matrix (DBM), which was found to have fair osteoinductive and osteoconductive properties in previous studies4,25,26). Numerous studies have reported superior or equivalent outcomes of DBM as a bone graft extender compared to autologous bone grafts alone1,17). However, evidence regarding whether DBM is sufficient as bone void filler in lumbar spinal fusion is still lacking.
Many host factors, including osteoporosis, hormone therapy, medications, nutrition and smoking status, can influence the bone graft, and the surgeon’s technique including the preparation of the fusion bed, instrumentation, and manipulation of bone graft, can affect the fusion rate2,18). An in vivo study performed in the same bone growth environment is required to determine the true fusion rate of DBM as bone void filler.
We present our prospective study of patients who underwent lumbar interbody fusion using both autologous bone grafts and DBM as bone void filler.
MATERIALS AND METHODS
Patient population
From April 2014 to August 2015, twenty patients with spinal stenosis were included in this prospective study. Patients between ages 20 and 75 who were diagnosed with lumbar spinal stenosis or spondylolisthesis and eventually required a one or two-level fusion surgery were included in the study. The exclusion criteria were as follows: having undergone revision surgery, trauma, compression fracture, presence of malignancy, or infectious disease. Clinical and radiographic data were collected in accordance with the regulations of the institutional review board at Korea University Ansan Hospital.
Clinical and radiographic data acquisition
Clinical outcomes were assessed using the Numeric Rating Scale (NRS) of leg and back pain and the Korean Oswestry Disability Index (K-ODI)12). To assess for successful fusion, the range of motion (ROM) of the operated segment was measured using the cobb angle from the lateral flexion-extension simple radiographs acquired at the pre-operative assessment and at 3 months, 6 months, and 1 year postoperatively. We performed dual-energy x-ray absorptiometry (DEXA) scans to see if the patient’s bone density influenced fusion.
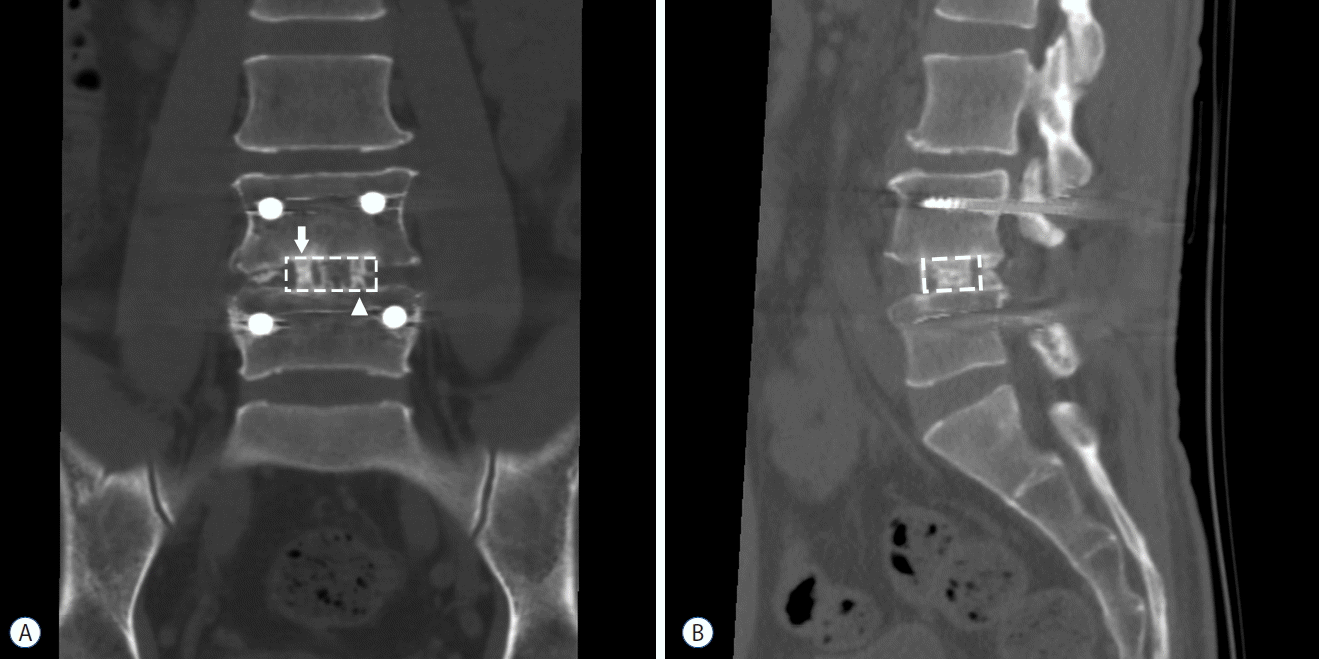
Computed tomography (CT) was performed at 1 year after fusion surgery to assess the amount of bone bridging at the graft sites. To quantify and objectively compare the amount of bone growth, we cropped the operated intervertebral space from the coronal and sagittal CT images, visualizing the center portion of the polyetheretherketone (PEEK) cage. From the coronal image, after inverting the white and black colors, we performed densitometric analysis on the white vertical band-like bone bridge inside the cages (Fig. 1A), using ImageJ 1.50i software (W. Rasband, National Institutes of Health, Bethesda, MD, USA; available at http://rsb.info.nih.gov/ij/). From the sagittal CT images, two mid-cage (autologous bone grafts and DBM) slices were collected from each patient (Fig. 1B). The white bone growth area inside the cage was measured by pixel calculation using ImageJ software.
Operative technique
During the operation, specifically during conventional posterior lumbar interbody fusion (PLIF), PEEK cages were used for interbody fusion to visualize bone growth on radiographs. After subtotal laminectomy and bilateral medial facetectomies, the local autologous bone chips were collected and the cancellous portion was used to fill the PEEK cage on the right side of the patient. After insertion of the cage on the right side, the remnant autologous bone chips were placed between the cages. Then, another PEEK cage filled with putty-type DBM (Bonfuse®, CGBio, Seoul, Korea) was inserted into the left intervertebral space. For every patient, pedicle screws were inserted and compression against the interbody cages followed.
Statistical analysis
Statistical analyses were performed with IBM SPSS statistics 20.0 software (IBM Corp., Armonk, NY, USA). The comparison between preoperative and postoperative parameters and between DBM and autologous bone grafts parameters were performed using paired t-tests, except for the assessment of the pixels from the sagittal view, which were compared using Wilcoxon signed rank test due to its lack of a normal distribution. The existence of an association among the numerical parameters was assessed by Pearson correlation and partial correlation analysis.
RESULTS
Of the initial 20 patients, 2 patients were excluded because of lack of 1-year follow-up. The remaining 18 patients consisted of 10 men and 8 women with a mean age of 56.4 (32–71). The operated level ranged from L3/4 to L5/S1. Eleven patients had single-level and 7 patients had two-level repairs. As shown in Fig. 2, all the mean values of the NRS and K-ODI clinical outcome improved at the 1-year postoperative assessment. The mean value of the back pain NRS improved from 4.61 to 2.78 (p=0.003), and that of leg pain NRS improved from 6.89 to 2.39 (p<0.001). The mean K-ODI score also improved from 27.33 to 13.83 (p<0.001). Age, sex, and the operated level were not significantly correlated with clinical outcomes.
The measured ROM of the operated segment dramatically decreased in all patients, from 7.75 degrees to 1.93 degrees (p<0.001). The ROM decreased below 2.0 degrees at the 3-month assessment, and remained less than 2 degrees through the 1 year postoperative assessment (Fig. 2D).
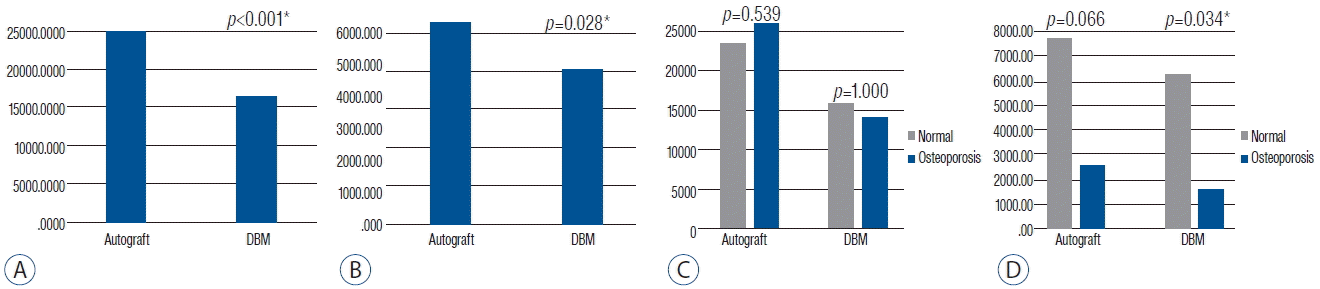
On the follow up CT scan at 1-year postoperative assessment, bone bridge formation inside the cage was observed in every patient regardless of whether the graft material was autologous bone or DBM (Fig. 1). On the quantitative comparison of bone growth, autologous bone graft showed a higher density of bone bridges on the coronal CT view (Fig. 3A, p<0.001) and a larger area on the sagittal CT view compared to DBM (Fig. 3B, p=0.028).
For bone density, the mean T-score was −0.96. With adjustment for age, which demonstrated a negative correlation with T-score (p=0.005), the partial correlation analysis did not show an association between T-score and bone growth. There were four osteoporotic patients whose T-score was under −2.5. Though densitometric analysis on coronal CT views did not show a significant difference based on osteoporosis (Fig. 3C), on sagittal CT views, both the autologous bone and the DBM side of osteoporotic patients showed much smaller bone growth areas than those of normal patients (1/4 and 1/3, p=0.066 and p=0.034, respectively) (Fig. 3D).
DISCUSSION
DBM is an acid extraction product of cadaver bone, which was first developed by Marshall Urist in 196527). While most bone graft substitutes and synthetics show osteoconductive property rather than osteoinductive property, DBM is known to have osteoinductive properties as well8,9,21). Though its demineralization process remove cells and structural strength from the cadaver bone, its remaining proteinaceous components enhance the bioactivity6,18,21,27). DBM is widely used in recent spine surgeries, and the most popular type of DBM graft is the putty type, which has the viscosity to prevent wash-out by irrigation9).
In lumbar posterolateral fusion, Cammisa et al. suggested that DBM can reduce the quantity of autologous bone grafts harvest and Morone et al. suggested that DBM was more effective when it was applied as a bone graft extender in posterolateral fusion3,19). Different from posterolateral fusion, which, according to Wolff’s Law5), cannot provide enough compression to the bone graft, we expect that DBM can be a promising alternative for autologous bone grafts in interbody fusion. Kim et al. reported similar fusion rates for DBM compared to autologous bone grafts as a bone void filler in lumbar interbody fusion cases13). In this study, we applied autologous bone grafts and DBM simultaneously and separately in each patient to control for host and surgeon factors. We found that the natural bone growth of autologous bone grafts using local bone was superior to that of DBM. The bone growth of the DBM side was 65% of the autologous bone grafts on densitometry and 77% in area by pixels (Fig. 3A, B). On the other hand, though it was not included in the analysis, even the autologous bone grafts, between the cages, showed favorable bone growth in most patients. This finding implies that spine surgeons can gain additional fusion beds by packing remnant bone chips to the disc spaces outside the cages.
A CT scan has superior sensitivity to show bone growth in the graft area compared to a simple radiograph15). Particularly, three–dimensional (3D) helical CT is very useful for visualizing the growing bone bridges15). In this study, we collected coronal and sagittal images of the patients to identify whether the cranial and caudal endplate is connected by vertical bone bridges (Fig. 1). In the interpretation of fusion using the CT scans, most previous studies have focused on the presence and continuity of bone bridges7,13,24). However, a simple classification based on presence or absence may have some limitations. Clinicians usually prefer to limit CT scans because of radiation exposure. When the CT scan follow-up is done too early or too late, it is difficult to reveal the different fusion rates between graft materials. In our series, on the one-year follow-up CT, 9 of 18 patients showed continuous bone bridges. Moreover, the remaining 9 patients still showed gaps between the cranial and caudal bone bridges. Among the 9 patients with continuous bridging, 3 patients showed bilateral bridging, 4 patients showed bridging only on the autologous bone graft side, and 2 patients had bridging on the DBM side. We could not find significant differences regarding the “continuity of bone bridges” between autologous bone grafts and DBM. However, the calculated pixels of the bone bridge were significantly higher in autologous bone grafts (Fig. 3).
Though some authors reported that a flexion–extension radiograph is a simple, favorable method to determine whether the patient’s operated level achieved solid fusion7), it is limited in its ability to identify a nonunion that has subtle motion15). Larsen et al. demonstrated that the stability of the pedicle screw can restrict meaningful motion even in patients with pseudarthrosis16). In our series, even in patients who showed gaps between the bridges, the ROM was maintained below 2 degrees during the 1-year follow up period (Fig. 2D). Assuming that modern spinal devices prevent obvious motion even in cases of nonunion, we suggest that a multidisciplinary assessment, including clinical examination, flexion–extension radiograph, and CT scan, is required to interpret “solid fusion” in lumbar interbody fusion.
A PEEK cage, due to its less stiff Young’s modulus, is known to have a superior load sharing effect than titanium cages22,28). However, it has an inferior osseointegration ability20,23). Therefore, solid fusion of the graft packed into the cage is mandatory to prevent pseudarthrosis. PEEK cages cause less artifact changes on a radiograph, so the radiolucent characteristics of the PEEK cage are very useful to assess the pattern of bone growth inside the cages7,22). In this study, we could easily identify the extent and continuity of the bone bridges inside the PEEK cages (Fig. 1).
Based on previous studies, osteoporosis impairs the bone healing process after fractures and can result in lower fusion rates in spine surgeries10,14,18). In this study, patients with osteoporosis showed less bone formation in both autologous bone grafts and DBM (Fig. 3D) compared to patients without osteoporosis. The bone growth area in the DBM packed cage was significantly smaller (1: 4) in osteoporosis patients (Fig. 3D). Though none of the 4 osteoporotic patients showed pseudarthrosis during 1-year follow up, we recommend autologous bone grafts for patients with osteoporosis, and harvesting of the iliac crest should be considered as well.
The strength of our study is that we observed the simultaneous natural bone growth rate of autologous bone grafts and DBM in vivo and in same environment, and performed analysis using enumerated data obtained from CT images and image calculation software. However, there are some limitations. Some comparative analyses failed to reach statistical significance due to the small population size, particularly in the osteoporosis cohort. Another limitation was that our patients received autologous bone grafts and DBM at the same time. Therefore, we could not evaluate pure clinical outcomes of DBM alone. Our follow-up period was 1 year, which may have underestimated the development of pseudarthrosis and instrument failure. Patients who showed incomplete bridging during the first year require further follow up to determine whether they will develop solid fusion or pseudarthrosis.
CONCLUSION
Both local autologous bone grafts and DBM can induce favorable bone growth independently in lumbar interbody fusion. If it is available, DBM should be used as an autologous bone graft extender. In situations where access to autologous bone grafts is limited, such as minimally invasive surgery, DBM can be used as bone void filler. In osteoporotic patients, DBM is recommended as a graft extender, not bone void filler, and an iliac crest harvest should be considered.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download