INTRODUCTION
MATERIALS AND METHODS
Animals
Experimental design
 | Fig. 1Apomorphine rotation test. This graph showed that the mean and 95% conÿdence interval in 6-OHDA group (134.3, 114.80 to 153.86) and 6-OHDA plus DBS group (127.66, 101.98 to 153.35). There was no statistical signiÿcance of radiation turns between two groups (p=0.6069). 6-OHDA: 6-hydroxydopamine, DBS: deep brain stimulation. |
6-OHDA lesions
Surgery and microdialysis procedures
STN stimulation
Analytical procedures
Tyrosine hydroxylase (TH) immunohistochemistry
Statistical analysis
RESULTS
Effect of STN-HFS on extracellular glutamate levels in the striatum of sham, 6-OHDA group, and 6-OHDA plus DBS group
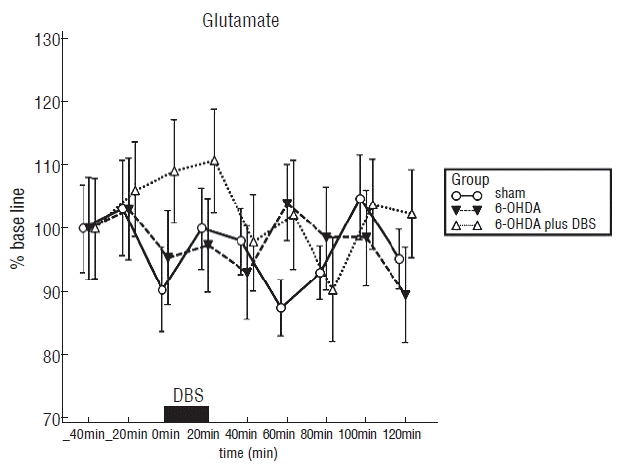 | Fig. 2E°ect of high frequency stimulation of subthalamic nucleus on extracellular glutamate in the striatum measured by microdialysis in rats. Each point represents the mean value±standard error from six rats per group: normal rats without deep brain stimulation (sham group), 6-OHDA-lesioned rats without deep brain stimulation group (6-OHDA group), 6-OHDA-lesioned rats with deep brain stimulation (6-OHDA plus DBS group). 6-OHDA: 6-hydroxydopamine, DBS: deep brain stimulation. |
The effect of STN-HFS on extracellular GABA levels in the striatum of sham, 6-OHDA group, and 6-OHDA plus DBS group
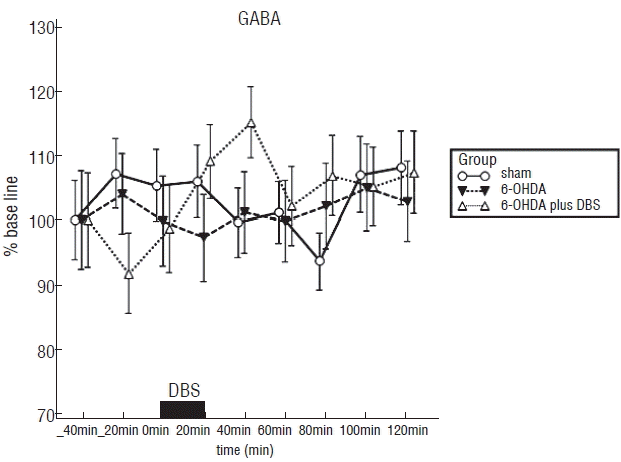 | Fig. 3Effect of high frequency stimulation of subthalamic nucleus on extracellular GABA in the striatum measured by microdialysis in rats. Each point represents the mean value±standard error from six rats per group: normal rats without deep brain stimulation (sham group), 6-OHDA-lesioned rats without deep brain stimulation group (6-OHDA group), 6-OHDA-lesioned rats with deep brain stimulation (6-OHDA plus DBS group). GABA: gamma-aminobutyric acid, 6-OHDA: 6-hydroxydopamine, DBS: deep brain stimulation. |
Histological controls
 | Fig. 4Cresyl violet stained photomicrographs showing the sites of deep brain stimulation in the subthalamic nucleus. Scale bar: 250 um. STN: subthalamic nucleus. |
 | Fig. 5Photographs of coronal rat brain sections at nigral and striatal levels. Sham group: tyrosine hydroxylase (TH) immunostaining at nigral and striatal levels has no di°erence between ipsilateral and contralateral side. 6-OHDA group: TH immunostaining at nigra and striatum in ipsilateral side to 6-OHDA lesioned site shows remarkable decrease compared with contralateral side. 6-OHDA plus DBS group: TH immunostaining at nigra and striatum in ipsilateral side to 6-OHDA lesioned site shows mild increase compared with the ipsilateral side in 6-OHDA group. Scale bar=500 um. 6-OHDA: 6-hydroxydopamine, DBS: deep brain stimulation, ST: striatum, SN: substantia nigra, VT: ventral tegmental area. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download