This article has been
cited by other articles in ScienceCentral.
Abstract
Craniopharyngiomas exhibiting histologic malignancy are extremely rare. Herein, we report the case of a 26-year-old male patient who underwent suprasellar mass excision via an interhemispheric transcallosal approach. Histopathological examination indicated that the craniopharyngioma was of the adamantinomatous subtype. The patient received postoperative medical treatment for endocrine dysfunction and diabetes mellitus without radiation treatment. Two years after the operation, he presented with progressive visual disturbance and altered mentality. Magnetic resonance imaging revealed a huge mass in the suprasellar cistern and third ventricle. He underwent a second operation via the same approach. The histopathological examination showed an adamantinomatous craniopharyngioma with sheets of solid proliferation in a spindled pattern, indicating malignant transformation. Malignant transformation of craniopharyngioma in the absence of radiation therapy has been reported in only five cases, including this one. We present a case of malignant transformation of craniopharyngioma with a brief review of relevant literature.
Go to :

Keywords: Craniopharyngioma, Malignant transformation, Radiotherapy
INTRODUCTION
Craniopharyngioma is a type of benign tumor that arises from Rathke’s pouch, a derivative of the oral ectoderm, and accounts for approximately 3–3.5% of all intracranial tumors
3). Although regarded as a world health organization (WHO) Grade I tumor, it often demonstrates aggressive local behavior after surgery and requires adjuvant radiotherapy. Craniopharyngiomas are known to recur even after presumed total resection, especially at the primary site or in its vicinity, suggesting microscopic residual disease. It has been estimated that the recurrence rates after total and subtotal resection are 30% and 50–70%, respectively. Thus, adjuvant radiotherapy following subtotal resection has been recommended
8).
Craniopharyngioma rarely undergoes malignant transformation, a phenomenon that was most likely first described by Salyer in 1973
10). The etiology and pathogenesis of malignant transformation is unclear, although previous literature has suggested a correlation with radiotherapy
9). The prognosis of malignantly transformed craniopharyngioma is poor and because of its low incidence rate, there is insufficient evidence for various treatment modalities. Herein, we report a case of craniopharyngioma with subsequent malignant transformation 2 years after the first operation.
Go to :

CASE REPORT
A 26-year-old male patient presented with a 1-week history of progressive headache and visual disturbance without any other neurological deficits. Brain magnetic resonance imaging (MRI) revealed a suprasellar mass lesion with enhanced solid and multiseptated cystic components measuring 3.0×2.8 cm (
Fig. 1). Preoperative investigations of pituitary function including prolactin and thyroid function did not reveal any abnormalities. The patient underwent an excision via an interhemispheric transcallosal approach with near-total resection of the tumor. The bulk of the tumor was noted to be solid and calcified. Histopathological examination revealed a typical adamantinomatous craniopharyngioma (
Fig. 2). There was no cytologic atypia and mitoses.
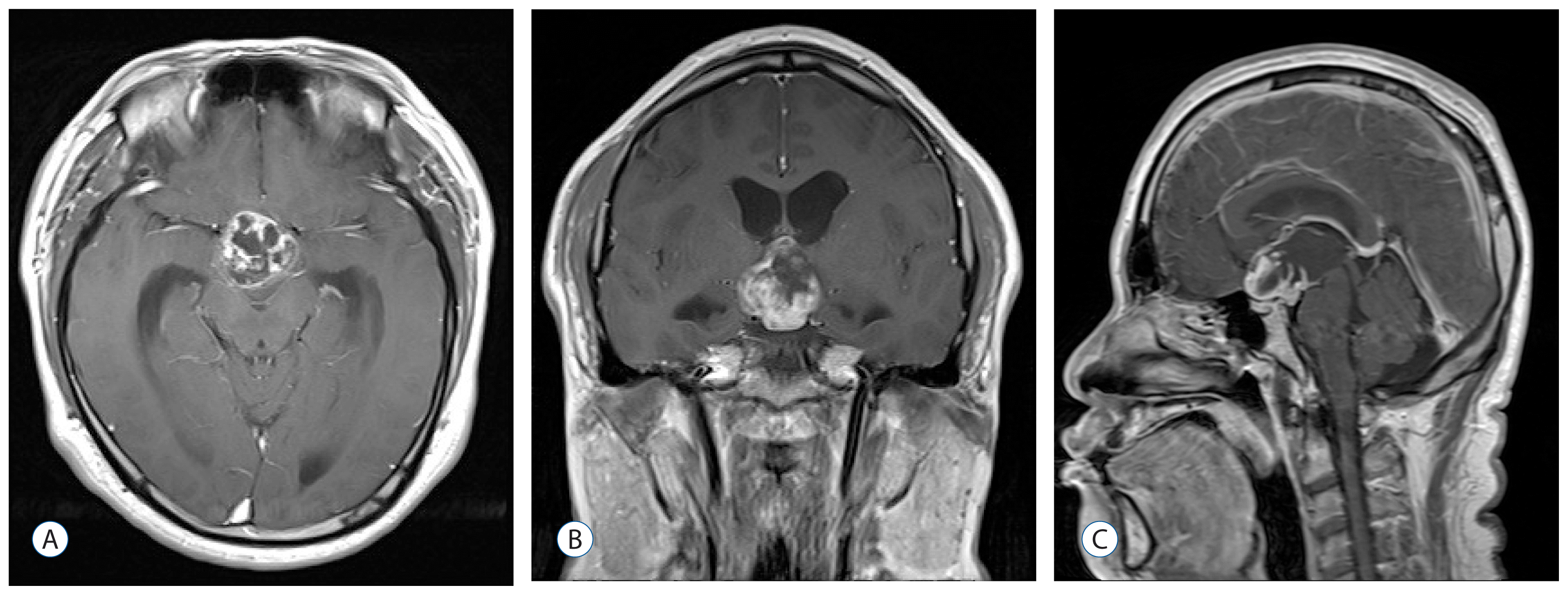 | Fig. 1Brain magnetic resonance imaging before the first surgery, showing a suprasellar mass lesion with enhanced solid and multiseptated cystic components. A : Axial. B : Coronal. C : Sagittal. 
|
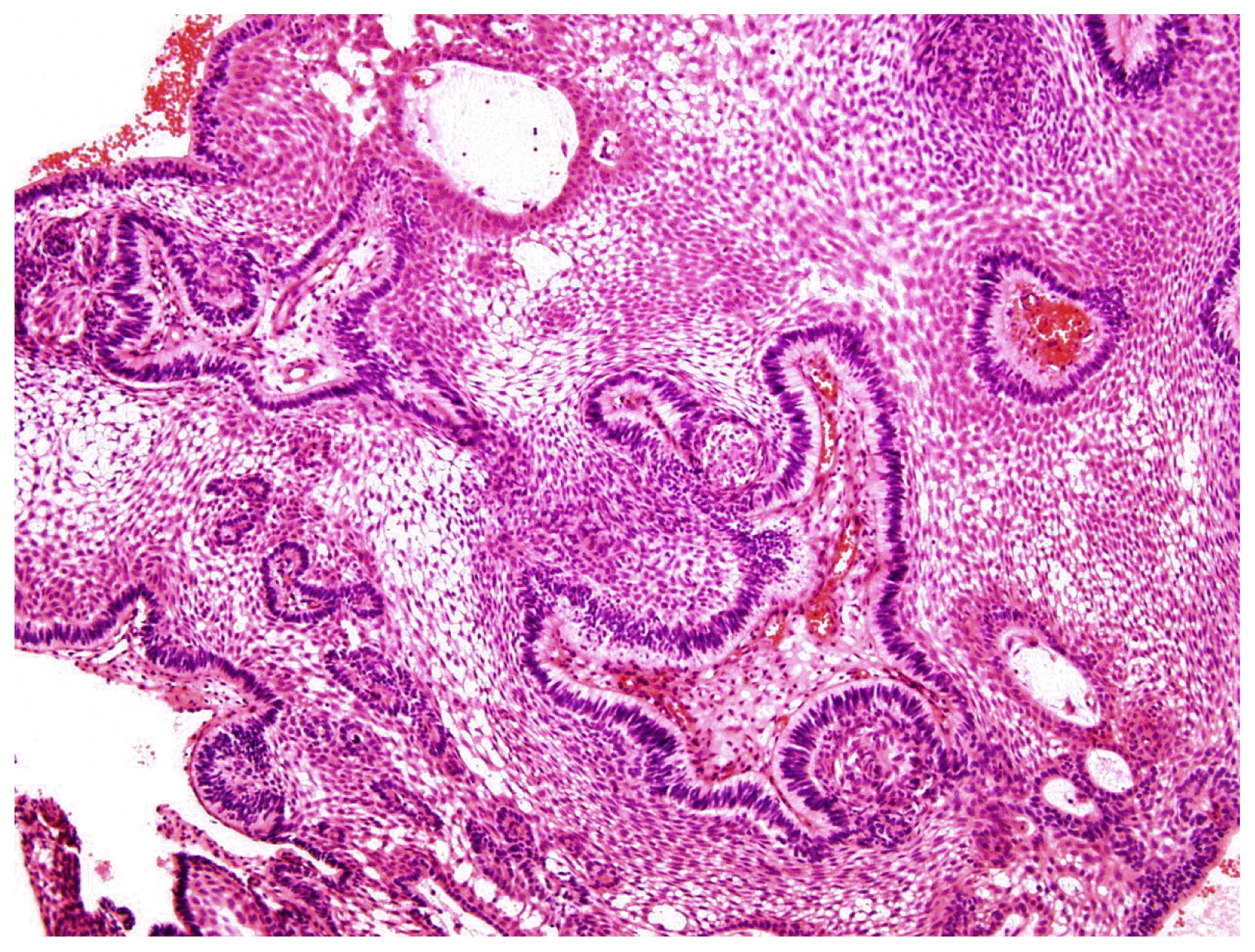 | Fig. 2The first biopsy showing a typical adamantinomatous craniopharyngioma. 
|
In the immediate postoperative period, the patient’s visual impairment remained unchanged. He experienced endocrine dysfunction characterized by hypopituitarism and diabetes insipidus.
After 6 months, MRI showed a residual mass enhanced along the right anterior margin of the third ventricle floor (
Fig. 3). The patient was treated with medication for endocrine dysfunction and refused radiation therapy for the residual tumor.
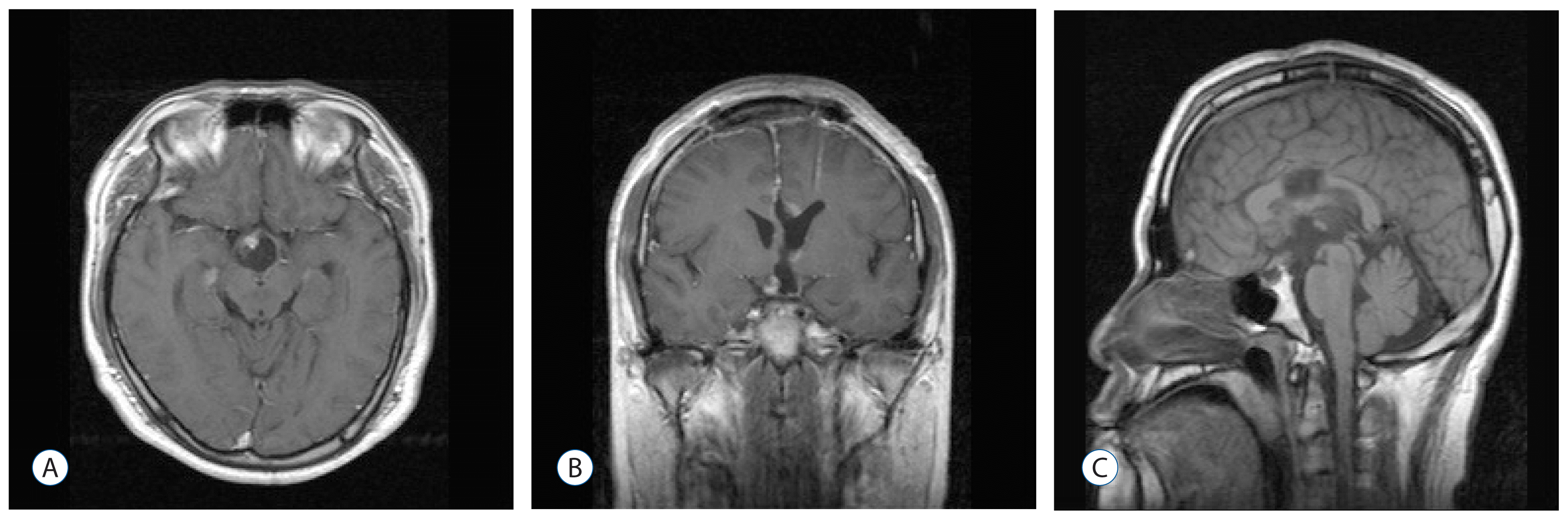 | Fig. 3Post-operative magnetic resonance imaging scan obtained 6 months after the first surgery, showing a small residual enhancing mass along the right anterior margin of the floor of the third ventricle. A : Axial. B : Coronal. C : Sagittal. 
|
Two years after his first operation, the patient presented to our department with confused mental status and progressive visual disturbance with bitemporal hemianopsia. MRI showed a recurrence of a multiseptated cystic mass measuring 4.7×5.0 cm in the suprasellar cistern, third ventricle, and parts of the lateral ventricles (
Fig. 4). He was operated on via an interhemispheric transcallosal approach along the previous incision, and gross total resection of the tumor was performed. Macroscopically, the tumor was soft and calcified with relatively defined boundaries compared to the surrounding tissue. Histopathological examination showed craniopharyngioma with cytologically malignant features. The tumor was highly cellular and presented with a spindled pattern (
Fig. 5A, B). Immunological tests showed overexpression of p53 and Ki67 (Ki67 labeling index 15%) (
Fig. 5C, D), and the tumor cells were positive for pancytokeratin and vimentin (
Fig. 5E, F).
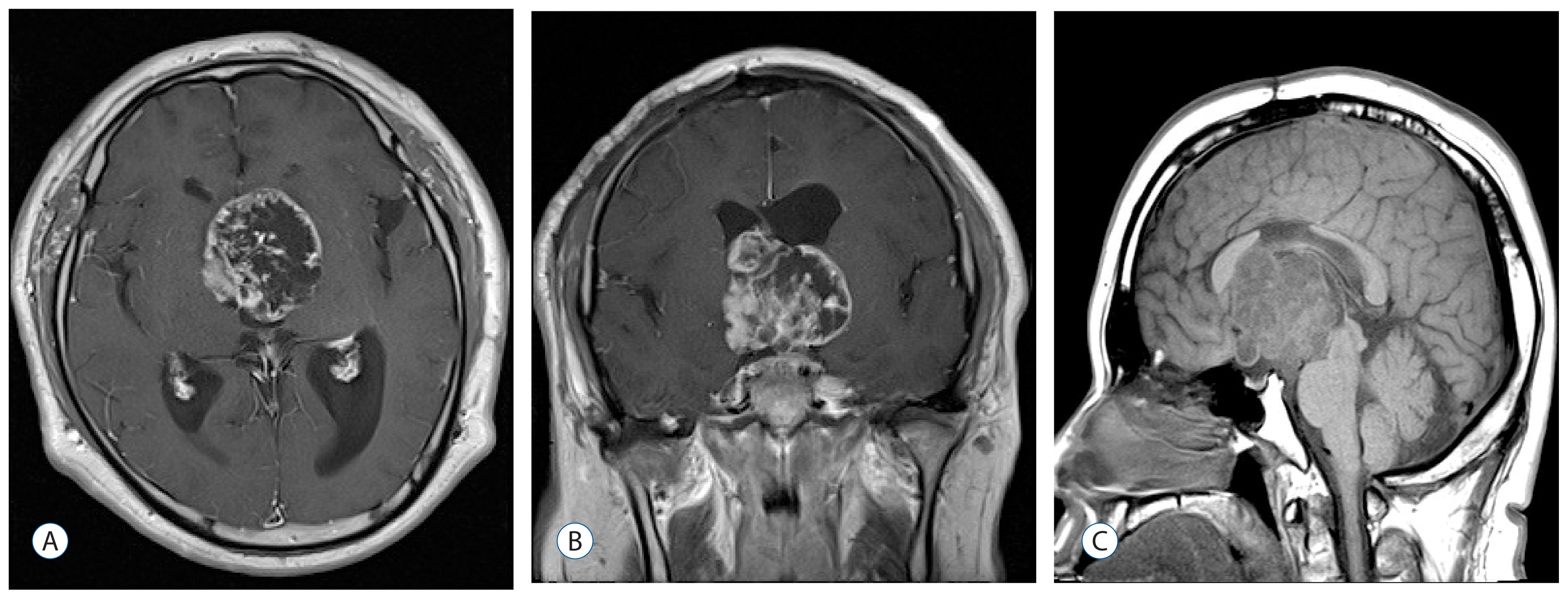 | Fig. 4Follow-up brain magnetic resonance imaging 2 years after the first surgery, showing recurrence of multiseptated huge cystic mass in the suprasellar, third, and lateral ventricles. A : Axial. B : Coronal. C : Sagittal. 
|
 | Fig. 5Histopathologic findings of a specimen obtained during the second surgery. Typical adamantinomatous craniopharyngioma that is highly cellular and presents with a spindled pattern (A, B; hematoxylin-eosin). The immunological tests showing overexpression of p53 (C) and Ki67 (D), and positive results for pancytokeratin (E) and vimentin (F). 
|
After the second operation, the patient’s symptoms of headache and impaired consciousness improved but the endocrine dysfunction and visual disturbances persisted. After three months, MRI showed no residual tumor (
Fig. 6). However, seven months after the second operation, he visited the emergency room (ER) with a high fever, general weakness, and drowsy mentality. The patient’s blood work revealed severe electrolyte imbalance and dehydration. While being evaluated in the ER, he showed a sudden drop in blood pressure and expired shortly after despite immediate efforts to resuscitate him. No autopsy was performed.
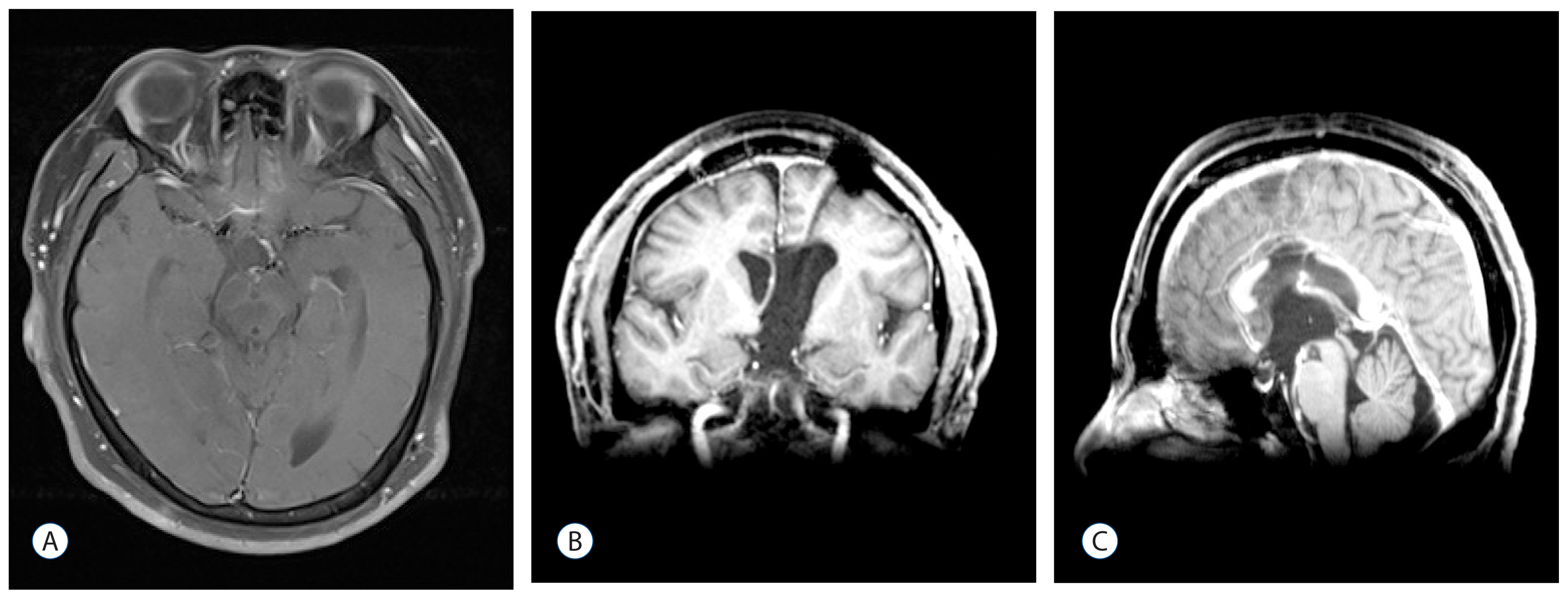 | Fig. 6Brain magnetic resonance imaging after the second surgery showing total resection of the recurrent mass. A : Axial. B : Coronal. C : Sagittal. 
|
Go to :

DISCUSSION
Malignant transformation of craniopharyngioma is extremely rare and is not included in the current WHO classification of tumors of the central nervous system published in 2007
7). Malignant craniopharyngiomas are presumably derived from the remnants of Rathke’s pouch, which was first described by Akachi in 1987
1). A total of 23 cases have been reported since then and only five cases exhibited malignant craniopharyngioma without radiotherapy. Four of these cases developed de novo (primary type) and only one case developed by malignant transformation of craniopharyngioma (secondary type) as in the present case (
Table 1).
Table 1
Clinical data for six cases of malignant craniopharyngioma without radiotherapy
|
Authors & Year |
Sex |
Age at onset (years) |
Period of transformation (years) |
Imaging examinations |
Histology at onset |
Histology upon transformation |
|
Yue and Da (2006)12)
|
M |
17 |
0*
|
Heterogeneously enhancing suprasellar mass |
Squamous cell carcinoma |
Squamous cell carcinoma |
|
Rodriguez et al. (2007)9)
|
M |
31 |
0*
|
Heterogeneously enhancing suprasellar mass with cystic components and calcification |
Odontogenic ghost cell carcinoma |
Odontogenic ghost cell carcinoma |
|
Boongird et al. (2009)2)
|
F |
46 |
0*
|
Heterogeneously enhancing sellar mass with necrosis |
Squamous cell carcinoma |
Squamous cell carcinoma |
|
Jaggon et al. (2009)5)
|
F |
54 |
0*
|
Heterogeneously enhancing suprasellar mass |
Squamous cell carcinoma |
Squamous cell carcinoma |
|
Gao et al. (2011)4)
|
F |
41 |
4 |
Heterogeneously enhancing sellar/suprasellar mass with cystic components and calcification |
Papillary craniopharyngioma |
Ameloblastic carcinoma |
|
Present case |
M |
26 |
2 |
Heterogeneously enhancing suprasellar mass with cystic components |
Adamantinomatous craniopharyngioma |
Sarcomatous transformation |

According to the literature, the pathologic findings of malignant craniopharyngiomas are heterogeneous including squamous cell carcinoma, myoepithelial carcinoma, ameloblastoma, ameloblastic carcinoma, and malignant craniopharyngioma mimicking odontogenic ghost cell carcinoma. A case of malignant craniopharyngioma with spindle cell proliferation that presented with sarcomatous transformation has been reported
11). Although diagnostic criteria for malignant craniopharyngioma have not yet been established, the following histology is suggestive of malignantly transformed craniopharyngioma: increased cellularity, nuclear pleomorphism, increased mitotic activity or Ki67 proliferation indices, necrosis, or vascular proliferation. Our case is similar to the case reported by Wang et al.
11) in that, the malignantly transformed portion was derived from the stromal spindle cell portion of the preexisting craniopharyngioma.
There are various causes of malignant transformation in craniopharyngioma; however, the exact mechanism is currently unknown because of the limited number of reported cases. According to the 13 case reports in the literature, radiotherapy might have an influence on malignant transformation. The occurrence of neoplasms after radiotherapy is well accepted
6).
Similar to our case of malignant transformation without radiotherapy, Gao et al.
4) reported histopathological confirmation of papillary craniopharyngioma after the patient’s first surgery, followed by histopathological confirmation of adamantinomatous craniopharyngioma with ameloblastic carcinoma due to regrowth of the mass in some areas after the second surgery. After one year, the patient underwent a third surgery owing to recurrence and histopathological examination showed clear malignant features.
In our case, the patient was diagnosed with a typical adamantinomatous craniopharyngioma during the first surgery; however, after the second surgery, histopathological examination showed malignant features and confirmed that the craniopharyngioma was of the adamantinomatous type. Therefore, other factors besides radiotherapy might contribute to malignant transformation of craniopharyngioma.
Go to :

CONCLUSION
Malignant transformation of craniopharyngioma in the absence of radiation therapy is extremely rare. The exact mechanism of the malignant transformation remains to be studied. Recurrence of craniopharyngioma within a short period, especially after radiation therapy, could result in its malignant transformation.
Go to :







 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download