1. Albuquerque FC, Park MS, Abla AA, Crowley RW, Ducruet AF, McDougall CG. A reappraisal of the Pipeline embolization device for the treatment of posterior circulation aneurysms. J Neurointerv Surg. 2015; 7:641–645. PMID:
25092926.

2. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms : results from a multicenter clinical trial. Radiology. 2013; 267:858–868. PMID:
23418004.

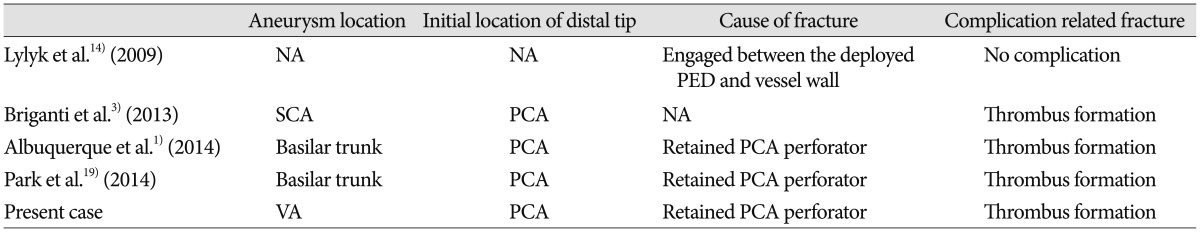
3. Briganti F, Marseglia M, Leone G, Briganti G, Piccolo D, Napoli M, et al. Endovascular treatment of a small aneurysm of the superior cerebellar artery with a flow-diverter device. A case report. Neuroradiol J. 2013; 26:327–331. PMID:
23859291.

4. Chalouhi N, Tjoumakaris S, Dumont AS, Gonzalez LF, Randazzo C, Starke RM, et al. Treatment of posterior circulation aneurysms with the pipeline embolization device. Neurosurgery. 2013; 72:883–889. PMID:
23407289.

5. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Hasan D, Pema PJ, Gould G. Spontaneous delayed migration/shortening of the pipeline embolization device : report of 5 cases. AJNR Am J Neuroradiol. 2013; 34:2326–2330. PMID:
23811979.

6. Chalouhi N, Zanaty M, Whiting A, Tjoumakaris S, Hasan D, Ajiboye N, et al. Treatment of ruptured intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2015; 76:165–172. PMID:
25549187.

7. Ding D, Liu KC. Microsurgical extraction of a malfunctioned pipeline embolization device following complete deployment. J Cerebrovasc Endovasc Neurosurg. 2013; 15:241–245. PMID:
24167807.

8. Fiorella D, Lylyk P, Szikora I, Kelly ME, Albuquerque FC, McDougall CG. Curative cerebrovascular reconstruction with the Pipeline embolization device : the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg. 2009; 1:56–65. PMID:
21994109.

9. Fiorella D, Woo HH, Albuquerque FC, Nelson PK. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008; 62:1115–1120. PMID:
18580809.

10. Hauck EF, Natarajan SK, Langer DJ, Hopkins LN, Siddiqui AH, Levy EI. Retrograde trans-posterior communicating artery snare-assisted rescue of lost access to a foreshortened pipeline embolization device : complication management. Neurosurgery. 2010; 67:495–502. PMID:
21099578.
11. Jabbour P, Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Randazzo C, et al. The Pipeline Embolization Device : learning curve and predictors of complications and aneurysm obliteration. Neurosurgery. 2013; 73:113–120. discussion 120. PMID:
23615106.
12. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device : a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015; 36:108–115. PMID:
25355814.

13. Keskin F, Erdi F, Kaya B, Poyraz N, Keskin S, Kalkan E, et al. Endovascular treatment of complex intracranial aneurysms by pipeline flow-diverter embolization device : a single-center experience. Neurol Res. 2015; 37:359–365. PMID:
25310354.

14. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device : the Buenos Aires experience. Neurosurgery. 2009; 64:632–642. discussion 642-643. quiz N6. PMID:
19349825.
15. Munich SA, Tan LA, Keigher KM, Chen M, Moftakhar R, Lopes DK. The Pipeline Embolization Device for the treatment of posterior circulation fusiform aneurysms : lessons learned at a single institution. J Neurosurg. 2014; 121:1077–1084. PMID:
25192476.

16. Navarro R, Yoon J, Dixon T, Miller DA, Hanel RA, Tawk RG. Retrograde trans-anterior communicating artery rescue of unopened Pipeline Embolization Device with balloon dilation : complication management. J Neurointerv Surg. 2015; 7:e7. PMID:
24510421.
17. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011; 32:34–40. PMID:
21148256.

18. Oh SY, Kim MJ, Kim BS, Shin YS. Treatment for giant fusiform aneurysm located in the cavernous segment of the internal carotid artery using the pipeline embolization device. J Korean Neurosurg Soc. 2014; 55:32–35. PMID:
24570815.

19. Park MS, Albuquerque FC, Nanaszko M, Sanborn MR, Moon K, Abla AA, et al. Critical assessment of complications associated with use of the Pipeline Embolization Device. J Neurointerv Surg. 2015; 7:652–659. PMID:
24968879.

20. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery : the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010; 31:1139–1147. PMID:
20150304.

21. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms : midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; 265:893–901. PMID:
22996749.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download