Abstract
A 35 years old woman presented with an acute meningeal syndrome following an intra ventricular haemorrhage without subarachnoid haemorrhage. The angiography demonstrated a 6 mm partially thrombosed saccular aneurysm at the plexal point of the right anterior choroidal artery (AChoA). It was surgically approached inside the ventricle through a trans-temporal corticotomy. The aneurysm was excised after distal exclusion of the feeding artery under motor-evoked potentials monitoring. Of the 19 cases of distal AChoA aneurysm neurosurgical treatment, this is the only one performed under electrophysiology monitoring, a simple and safe method to detect and prevent motor tract ischemia. We discuss this rare case, along with a comprehensible review of the literature of the previous surgical cases of distal AChoA aneurysms.
Anterior choroidal artery (AChoA) aneurysms (AChoAA) are rare and count for less than 5% of all intracranial aneurysms. Most of the time, AChoAA arise from the posterior wall of the internal carotid artery (ICA) and are located at the proximal segment near the origin10). Distal AchoAAs (DAA) are even more exceptional with only 46 cases reported in the literature2). It is important to recognize such a peculiar location regarding specific problems raised for the treatment. In this report we describe for the first time the usefulness of motor-evoked potentials (MEP) to monitor and prevent post-operative motor impairment in distal anterior choroidal artery (AChoA) aneurysm surgery.
A 35 years old woman, non-smoker, with no past medical history was admitted in emergency for acute headache and vomiting compatible with an acute meningeal syndrome. Clinical examination showed drowsiness, mild neck stiffness but no focal neurological sign (WFNS II). However, her level of consciousness worsened until coma for which she was subsequently intubated.
A computed tomography (CT) demonstrated a pure intra ventricular haemorrhage (Fisher IV), with no associated subarachnoid haemorrhage and no visible vascular malformation on the CT angiography (CTA). An external ventricular drain (EVD) was inserted in emergency to treat the obstructive hydrocephalus. A digital subtraction angiography (DSA) failed to highlight the source of the bleeding. The patient was transferred to the neurosurgical intensive care unit and progressively wakened up. Eight days after her admission, a second vascular imagery workup spotted a left saccular DAA (Fig. 1A, B). Reviewing the first vascular images (CTA and DSA), the aneurysm was already present but barely visible as mostly thrombosed.
Treatment options were discussed with the neuroradiologists and a neurosurgical approach was chosen regarding the small diameter of the AChoA and the distal location of the aneurysm, making an endovascular option not suitable.
A standard temporo-pterional bone flap was cut and, under neuronavigation guidance, a trans-sulcal approach between the superior and the middle temporal gyrus (T1–2) was performed to access the left temporal horn. Inside the ventricle, a partially thrombosed, 6 mm in diameter aneurysm was found (Fig. 2A, B). Regarding the high ratio aneurysm size/AChoA diameter, a standard neck clipping was not achievable (Fig. 2C, D). Therefore it was decided to excise the aneurysm en bloc with sacrifice of the distal part of the AChoA. Before, a temporary clipping of the AChoA was realized just upstream the aneurysm under monitoring of MEP (Fig. 2E, F). After 10 minutes without any modification of recording, the left distal portion of AChoA was definitively occluded with 2 Weck® clips (Horizon™, TFX Medical, Athlone, Ireland) and the aneurysm removed (Fig. 2G, H). Clots inside the lateral horn were also evacuated. The procedure went uneventful and the patient recovered gradually with neither post-operative focal neurology nor hydrocephalus.
Histology confirmed the diagnosis of partially thrombosed (Fig. 1C) true aneurysm with an area of recent rupture (Fig. 1C). We found no cause of increased hemodynamic stress within the left AChoA which can contribute to the formation and rupture of this aneurysm type. It was therefore classified as idiopathic. Post-operative DSA was unremarkable. One year after the surgery, she remains well [Glasgow Outcome Scale (GOS) V].
The AChoA arises from the infero-lateral wall of the internal carotid artery, mostly as a single vessel, and runs posteriorly in the crural cistern in close association with the optic tract. Passing through the ambient cistern it enters the choroidal fissure to reach the choroidal plexus of the temporal horn. Paradoxically to its tiny mean outer diameter of only about 0.70 mm, it has an important functional role. Even though the AChoA vascular territory shows large variations among individuals, the invariable terminal fields of supply of this artery are the posterior two thirds of the posterior limb of the internal capsule, most of the globus pallidus, the beginning of the optic radiation, the lateral aspect of the lateral geniculate body, and the middle third of the crus cerebri. Therefore, occlusion of these branches generally results in various neurological problems, which include contralateral hemiplegia, hemianesthesia and, hemianopia. However, the blood supply from the plexal segment feeds mainly the choroid plexus extending from the temporal horn to the atrium and usually has anastomosis with the lateral posterior choroidal artery on the surface of the choroid plexus.
DAA are rare vascular abnormalities and moyamoya disease is associated in 54%. The number of reported cases of moyamoya disease associated with peripheral aneurysms has been increasing, and some hypotheses as to the mechanism of peripheral aneurysm formation have been offered.
Among 46 DAA cases published, 21 were operated with 2 failed surgeries, 9 were treated by an endovascular procedure, 13 were managed conservatively and for 3 the treatment is not given.
Clinical presentation of DAA rupture is often typical of an acute meningeal syndrome±focal neurological signs. The first reported case presented with symptoms of an obstructive hydrocephalus and a paediatric case was revealed by a hemiplegia following an ischemic stroke of the globus pallidus. However CT scan images may be misleading as the bleeding is more likely to be intra-ventricular or intra-parenchymal rather than in the subarachnoid spaces. Vascular imagery diagnosis of DAA can also be difficult regarding their unusual location, their small size or thrombosed sac.
Treatment options of DAA are mainly surgical regarding the AChoA gauge making difficult its endovascular catheterisation.
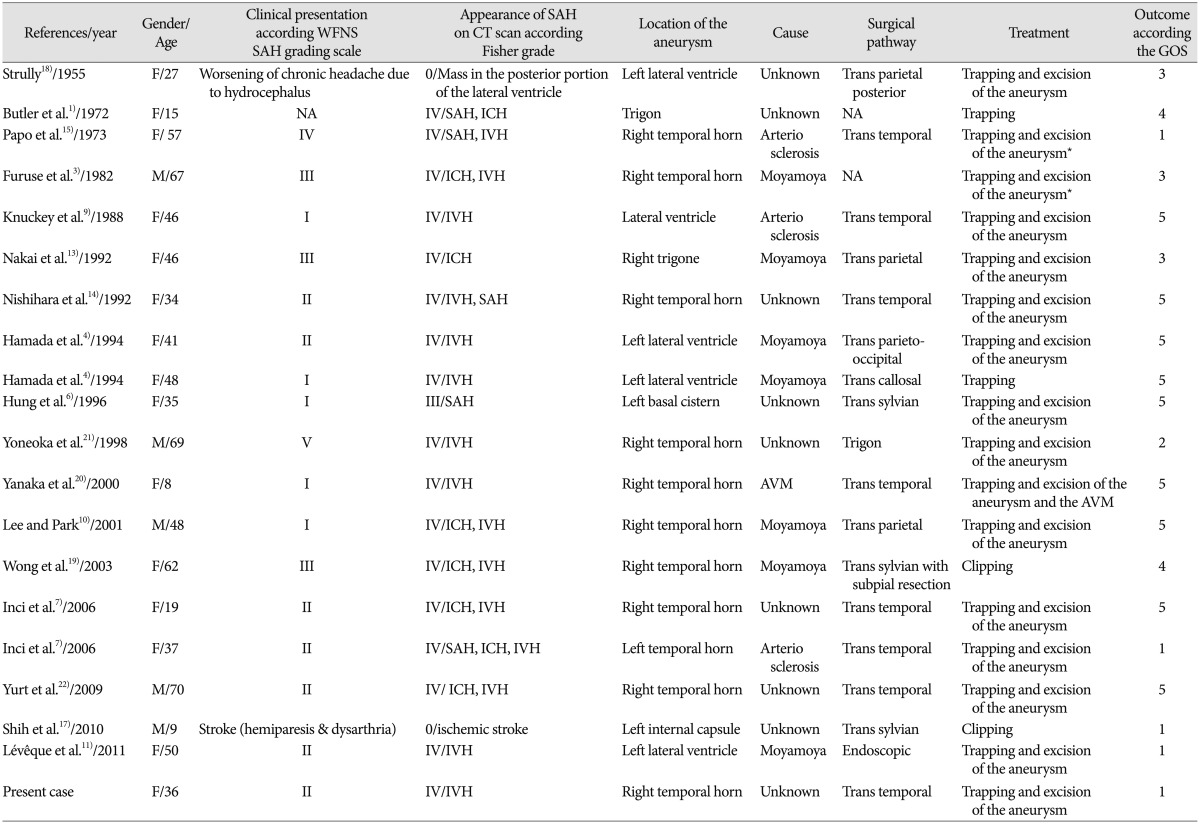
We review the 19 published surgical cases. The clinical features including clinical presentation according the WFNS SAH grading scale, appearance of subarachnoid haemorrhage on CT according the Fisher grade, location of aneurysm, cause, surgical pathway treatment and, outcome according the GOS, including ours are summarized in Table 1.
The location of the DAAs was in the temporal horn of the lateral ventricle, the so-called plexal segment, in the largest number (66.7%). Trapping of the parent artery and excision of the aneurysm was the most common surgical procedure applied as, like in our case the ratio aneurysm size on AChoA diameter was not suitable for clipping. Only in four cases, the authors report a successful clipping of the aneurysm with preservation of the feeding artery17192122). Regarding the localization of the DAA, different surgical pathways have been described. Via a trans-temporal/trans-parietal way for DAA located inside the ventricles or through a trans-sylvian/trans-choroidal fissure approach which allows proximal control of the parent vessel before aneurysm dissection. Image guided surgery with vascular sequences fused with parenchymal (MRI) images can be useful for a more accurate DAA targeting, especially for those located inside the ventricle. An important aspect of DAA surgery, in contrast to that for proximal aneurysm, is considered to be approaching the aneurysm with minimal brain damage, as most of them are reached through a trans-cerebral way. Endoscopic procedure can be achieved in case of intra-ventricular preferentially unruptured aneurysms11). In two reported cases, the surgical treatment was unsuccessful. In the first one the aneurysm could not be identify during the surgery. In the second case reported by Kasamo et al.8) the clip could not be applied on the aneurysm due to many moyamoya vessels surrounding the aneurysm, on contrary to what wrote some previous authors which didn't most likely read the original article and copy mistake of previous authors2).
Intra-operative ischaemic events during intracranial aneurysms clipping are the leading cause of permanent post-operative morbidity and mortality1016. MEP, recorded from muscles following motor cortex trans-cranial electrical stimulation, are commonly used for intra operative monitoring of pyramidal tract functional integrity. Therefore, they are useful for aneurysms which are supplied by arteries involved in pyramidal tract vascularization101216). Among them, AChoA has a particular significance as its first branches which rise from the cisternal segment, generally supply the posterior limb of the internal capsule that contains cortico-spinal fibers.
Surgical treatment of proximal AChoA aneurysms is known to involve a significant risk of vascularization insufficiency. AChoA branches have a variable origin that need better to be investigate before a surgical procedure. But due to their tiny diameters, DSA is often not enough accurate to pinpoint them, especially those reaching the internal capsule. Obliteration of a normal AChoA beyond the plexal point, where the artery enters the choroid plexus, is usually considered relatively safe. However this is not the case of patients with abnormal (moyamoya) AChoA providing collateral vessels to a large territory of the surrounding brain tissue. Thus, it is important to assess the functional effect of artery occlusion in order to adapt the surgical procedure. Intra operatively, this can be easily done by MEP monitoring.
The absence of branches to cerebral tissue after the anterior choroidal artery's ventricular penetration should allow trapping of the very peripheral aneurysm. However, this may be difficult to verify per operatively due to the limited surgical exposure. Sacrifice of AChoA's distal portion, even if constant anastomosis exist with the vertebro-basilar system, especially the postero-lateral choroidal and posterior cerebral artery, may lead to a stroke. This risk can be minimized by careful effort to preserve the AChoA patency based on AChoA-avoiding clip placement and intra operative monitoring. MEP modifications precede the occurrence of an irreversible ischaemic lesion and may provide the basis for immediate corrective procedures. Significant alteration of the MEP after temporary clamping is likely due to ischaemia and should thereby prevent a definitive vessel occlusion. In this last situation where the blood flow must be preserve, a non-deleterious treatment must be achieved. If a proper neck clipping can't be done, wrapping of the aneurysm may be a solution. Risking a post-operative hemiplegia, especially in a pre-operative non disabled a patient, is not acceptable. Regarding the diameter of the AChoA and the neck shape, an endovascular procedure may be attempt subsequently if the neurosurgical procedure failed to prevent safely a risk of re bleeding. On contrary, no MEP modification has a strong value for uneventful post-operative state. Other intra operative neurophysiological monitoring like somato-sensory evoked potentials can also be studied in the same goal.
Even if MEP is a well known tool, none of the 19 previous surgical cases mentioned the use of them per operatively. Although many authors have trapped the plexal segment and resected these aneurysms without any neurologic damage, we think that, whenever possible, this artery should be preserved.
Due to techniques improvements, more and more DAA are likely to be treated by endovascular procedure during which MEP study has also shown its usefulness5).
Outcomes of the published surgical cases were mainly dependant of pre operative state. Patients with severe clinical presentation due to an important intracerebral bleeding were more likely to be dead or highly disabled after neurosurgical treatment.
Nowadays, surgical aneurysms are usually the most complicated to treat. MEP monitoring is useful to prevent ischaemic stroke and severe post surgical outcome, at a time where the vascular neurosurgeons ought to keep post operative complications as low as possible relatively to the embolization.
DAA are exceptional and may be difficult to diagnosed, as they often present with intra ventricular±intra cerebral haemorrhage. Surgical approaches of DAA are different from proximal AChoA aneurysms and their complex management rerequired careful surgical planning for which per operative motor-evoked potentials monitoring is of great value in order to prevent catastrophic outcome and safely assume that the distal part of the AChoA can be safely occluded if necessary.
References
1. Butler AB, Partain RA, Netsky MG. Primary intraventricular hemorrhage. A mild and remediable form. Neurology. 1972; 22:675–687. PMID: 4673249.

2. Dolati P, Sutherland G, Wong J, Hudon M, Goyal M. Distal anterior choroidal artery aneurysm following iatrogenic posterior cerebral artery occlusion : a case report and review of literature. Acta Neurochir (Wien). 2012; 154:53–57. PMID: 22068715.

3. Furuse S, Matsumoto S, Tanaka Y, Ando S, Sawa H, Ishikawa S. [Moyamoya disease associated with a false aneurysm--case report and review of the literature]. No Shinkei Geka. 1982; 10:1005–1012. PMID: 7177326.
4. Hamada J, Hashimoto N, Tsukahara T. Moyamoya disease with repeated intraventricular hemorrhage due to aneurysm rupture. Report of two cases. J Neurosurg. 1994; 80:328–331. PMID: 8283274.

5. Hiraishi T, Fukuda M, Oishi M, Nishino K, Shinbo J, Sorimachi T, et al. Usefulness of motor-evoked potential monitoring during coil embolization of anterior choroidal artery aneurysms : technical reports. Neurol Res. 2011; 33:360–362. PMID: 21535934.

6. Hung KS, Lee TC, Lui CC. Aneurysm of superior branch of anterior choroidal artery mimicking carotid bifurcation aneurysm-case report. Acta Neurochir (Wien). 1996; 138:1464–1467. PMID: 9030355.

7. Inci S, Arat A, Ozgen T. Distal anterior choroidal artery aneurysms. Surg Neurol. 2007; 67:46–52. discussion 52. PMID: 17210297.

8. Kasamo S, Asakura T, Yamamoto Y, Kobayashi E. Unilateral moyamoya disease associated with multiple aneurysms. A case report and review of the literature. Neurol Med Chir (Tokyo). 1984; 24:30–34. PMID: 6204236.
9. Knuckey NW, Epstein MH, Haas R, Sparadeo F. Distal anterior choroidal artery aneurysm : intraoperative localization and treatment. Neurosurgery. 1988; 22(6 Pt 1):1084–1087. PMID: 3419573.

10. Lee YS, Park J. Anterior choroidal artery aneurysm surgery : ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 2013; 54:86–92. PMID: 24175021.

11. Lévêque M, McLaughlin N, Laroche M, Bojanowski MW. Endoscopic treatment of distal choroidal artery aneurysm. J Neurosurg. 2011; 114:116–119. PMID: 20540628.

12. Morandi X, Brassier G, Darnault P, Mercier P, Scarabin JM, Duval JM. Microsurgical anatomy of the anterior choroidal artery. Surg Radiol Anat. 1996; 18:275–280. PMID: 8983106.

13. Nakai H, Yamamoto K, Sako K, Tanikawa R, Kunimoto M, Hashimoto M, et al. [A ruptured aneurysm at the peripheral collateral circulation of the anterior choroidal artery in a patient with moyamoya disease : a case report]. No Shinkei Geka. 1992; 20:985–990. PMID: 1407365.
14. Nishihara J, Kumon Y, Matsuo Y, Sakaki S. A case of distal anterior choroidal artery aneurysm : case report and review of the literature. Neurosurgery. 1993; 32:834–837. discussion 837. PMID: 8492860.
15. Papo I, Salvolini U, Caruselli G. Aneurysm of the anterior choroidal artery with intraventricular hematoma and hydrocephalus. Case report. J Neurosurg. 1973; 39:255–260. PMID: 4719704.

16. Penchet G, Arné P, Cuny E, Monteil P, Loiseau H, Castel JP. Use of intraoperative monitoring of somatosensory evoked potentials to prevent ischaemic stroke after surgical exclusion of middle cerebral artery aneurysms. Acta Neurochir (Wien). 2007; 149:357–364. PMID: 17380251.

17. Shih P, Pinnaduwage T, Hu LS, Spetzler RF. A pediatric patient with a dissecting thrombotic anterior choroidal artery aneurysm : case report. Neurosurgery. 2010; 67:E518. PMID: 20644385.
18. Strully KJ. Successful removal of intraventricular aneurysm of the choroidal artery. J Neurosurg. 1955; 12:317–321. PMID: 14381939.

19. Wong GK, Boet R, Poon WS. Ruptured distal anterior choroidal artery aneurysm presenting with right intracerebral haematoma : clipping aided by subpial uncal resection. J Clin Neurosci. 2003; 10:689–691. PMID: 14592620.

20. Yanaka K, Tsuboi K, Fujita K, Aoki K, Takeuchi S, Anno I, et al. Distal anterior choroidal artery aneurysm associated with an arteriovenous malformation. Intraoperative localization and treatment. Surg Neurol. 2000; 53:546–551. PMID: 10940421.

21. Yoneoka Y, Ezuka I, Takai N, Oda T, Tamura T, Yamashita S. Ruptured distal anterior choroidal artery aneurysm presenting with casting intraventricular haemorrhage. Acta Neurochir (Wien). 1998; 140:185–189. PMID: 10399000.

22. Yurt A, Turan Y, Uçar K, Camlar M, Oran I. Ruptured distal anterior choroidal artery aneurysm. J Clin Neurosci. 2009; 16:132–134. PMID: 19013815.

Fig. 1
A : Left carotid artery digital subtraction angiography, lateral images showing the distal anterior choroidal artery aneurysm (black arrow). B : Zoomed, anterior-lateral and selective view of the aneurysm. C : Hematoxylin-eosin-saffron staining ×4 of the aneurysm showing the lumen (*), the thrombus (black arrow) inside the aneurismal wall which is ruptured (white arrow).
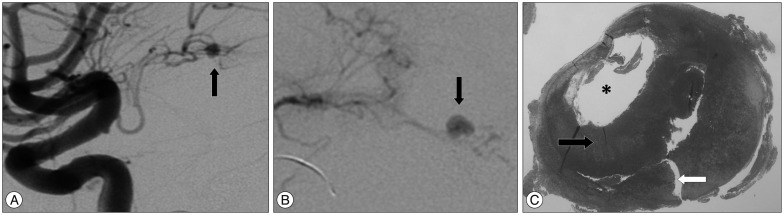
Fig. 2
A : Per operative view after trans sulcal approach between the superior and the middle temporal gyrus showing the intra ventricular hematoma (black arrow). B : Removal of the clot making the ependyma (black star) and the choroid plexus (black arrow) visible. C and D : exposition of the aneurysm (white arrow) and the distal AChoA (black arrow). E and F: temporary clipping (gray arrow) of the AChoA (black arrow). G and H : definitive Weck clip (gray arrow) of the AChoA. AChoA : Anterior Choroidal Artery.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download