Abstract
Objective
Because elderly patients are undergoing more surgeries, the importance of postoperative cognitive impairment (CI) evaluations is rising, especially for spine surgery, which is related to subjective pain. We investigated the prevalence of undiagnosed CI among elderly patients who underwent spine surgery and the impact of CI on postoperative outcomes.
Methods
The preoperative cognitive statuses of 129 patients over 65 who underwent lumbar spine surgery from 2012 to 2014 were determined with the Mini-Mental State Examination, and patients with scores under 24 were diagnosed with CI. The patients were then divided into a CI group (n=49) and non-cognitive impairment (NCI) group (n=80).
Results
Among the 129 patients, 49 (38.0%) were diagnosed with CI, and 9 (7.0%) had severe CI. The age of the CI group (72.88±6.20 years) was significantly greater than that of the NCI group (69.96±4.53 years). In contrast, the postoperative visual analog scale scores and performance statuses did not differ significantly. However, postoperative delirium was more frequent and the hospital stay length was longer in the CI group compared with the NCI group (p<0.05).
Conclusion
A high prevalence of undiagnosed CI was discovered among elderly patients undergoing spine surgery. The existence of CI was associated with higher rates of postoperative delirium and prolonged hospital stays, which affected clinical outcomes. Thus, CI assessments should be included in preoperative evaluations of elderly patients prior to spine surgery.
Postoperative cognitive dysfunction (POCD), which is a common neurological complication that presents after surgery, has been reported in 33–83% of patients undergoing cardiovascular surgeries16) and 9.9–12.7% of patients undergoing non-cardiac surgeries910). We have previously reported that POCD occurs in 24.3% of patients being treated with spine surgeries12).
In order to assess POCD, cognitive impairment (CI) is assessed before the surgery in order to obtain a baseline. Hogue et al.5) have reported that 45% of female patients undergoing coronary artery bypass graft (CABG) surgery show preexisting cognitive impairment (PreCI), while Silbert et al.14) reported PreCI in 35% of their patients before elective CABG surgery. Recently, 20% of the patients who were scheduled for total hip joint replacement surgery showed PreCI1).
Although it is important to assess cognitive function before all surgeries, the existence of PreCI before degenerative lumbar spine surgery, which is used to treat subjective pain and functionality, has a particularly large impact on postoperative care and prevention and on the determination of the treatment plan, surgical methods, and extent of surgery.
The aim of our study was to investigate the prevalence of PreCI in patients undergoing degenerative lumbar spine surgery and to explore the effects of PreCI on the surgery and postoperative care.
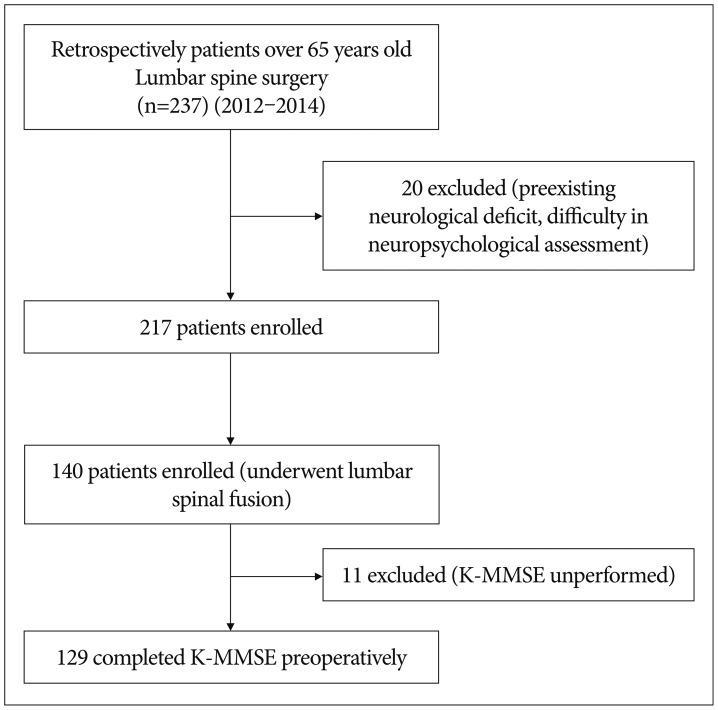
The subjects in this study were selected from among 237 patients who were over 65 years old and who underwent lumbar spine surgery between 2012 and 2014. Of these, we excluded patients with preexisting neurological deficits (e.g., stroke) and patients who were expected to have difficulties with the neuropsychological assessment (foreigner, blindness, deafness, etc.). Of the remaining 217 patients, 140 who underwent lumbar surgery for degenerative spine disease were registered for the study. After excluding 11 patients who were not able to perform the Korean Mini-Mental State Examination (K-MMSE), the remaining 129 patients were included in the final study (Fig. 1).
Cognitive function was measured with the K-MMSE prior to the surgery4). Scores of 24–30 were classified as no CI, 18–23 were mild CI, and under 17 were considered severe CI. The assessment was conducted in a quiet space within a general ward and without external disruption or interference. First, the patient or guardian (caregiver or relative) was interviewed about whether the patient had previously been diagnosed with dementia or if they had discovered any memory problems. Next, it was reconfirmed in a clinical record review whether the patient had any prior diagnosis or medical record that might indicate dementia or CI. All of the cognitive assessments were conducted by trained assessors.
For data collection, the patients were divided into two groups according to the presence or absence of CI. Baseline demographic data was collected through the review of the clinical records. The pre-operative data included age, sex, marital status, smoking, alcohol, comorbid conditions, education background, and preadmission medication. In addition, surgical type and laboratory abnormalities, such as complete blood count and metabolic panel (e.g., sodium, blood urea nitrogen, creatinine, albumin) were investigated. Prior to the surgery, the Eastern Cooperative Oncology Group (ECOG) performance status was used as a scale to assess the patients' mental state and level of physical activity15). Because the presence of pain may affect performance during cognitive testing, this was assessed with a visual analog scale (VAS). Finally, we investigated the length of time from when the patients were admitted to the hospital to the start of the surgery.
For the postoperative data, we investigated postoperative complications, including delirium, myocardial infarction, and urinary tract problems. In addition, we collected data on the presence or absence of re-operation, changes in the surgical plan, surgery time, discharge medication, and length of stay. The ECOG performance status was used to asseass the patients' mental states and physical activities after surgeries, and the VAS was used to assess pain. Finally, postoperative delirium was diagnosed with the Confusion Assessment Method13).
For the three groups, Student's t-tests, Mann-Whitney U-tests, and chi-square tests were used to test the comparisons. p values less than 0.05 were considered statistically significant. The statistical analyses were performed with SPSS software version 18.0 (IBM Corporation, Armonk, NY, USA).
The cognitive assessments were performed on each of the 129 patients, and 49 patients (38% of the total) were determined to have CI group. Within the CI group, 40 patients had K-MMSE scores of 18–23, which indicated mild CI, and 9 patients had K-MMSE scores below 17, which indicated severe CI (Table 1).
We compared the patient characteristics between the CI group and the non-cognitive impairment (NCI) group. The mean age of the CI group was 72.88±6.20 years old, and that of the NCI group was 69.96±4.53 years old, which indicated that the CI group was significantly older (p=0.005). The sex ratio (M : F) for the CI group was 14 : 35, and that for the NCI group was 37 : 43, with no significant difference between the groups. Marital status, smoking, and alcohol showed no significant differences between the groups (p>0.05) (Table 2).
We investigated the comorbid conditions prior to surgery. Although we found a number of conditions, such as hypertension, cardiovascular disease, diabetes mellitus, asthma, renal disease, and cancer, there were no statistically significant differences between the CI group and the NCI group. Preoperative ECOG performance status, presurgical medication, surgical method, and laboratory abnormalities did not differ between the groups (p>0.05). The time from hospital admission to the start of surgery was 4.59±4.59 days for the CI group and 3.82±7.01 days for the NCI group, which again showed no statistically significant difference (p>0.05).
We also investigated the patients' educational background. Education level was the duration of education that the patients received, and it was classified as less than 6 years, 6–9 years, 9–12 years, and 12 years or more. With these categories, the CI group showed 30, 10, 8, and 1 patients, respectively, while the NCI group showed 23, 19, 27, and 11 patients, respectisvely, which confirmed that the NCI group had a significantly higher educational background (p=0.001). The presurgical VAS scores were 6.82±1.20 for the CI group and 6.70±0.83 for the NCI group, which was not a significant difference (p>0.05) (Table 2).
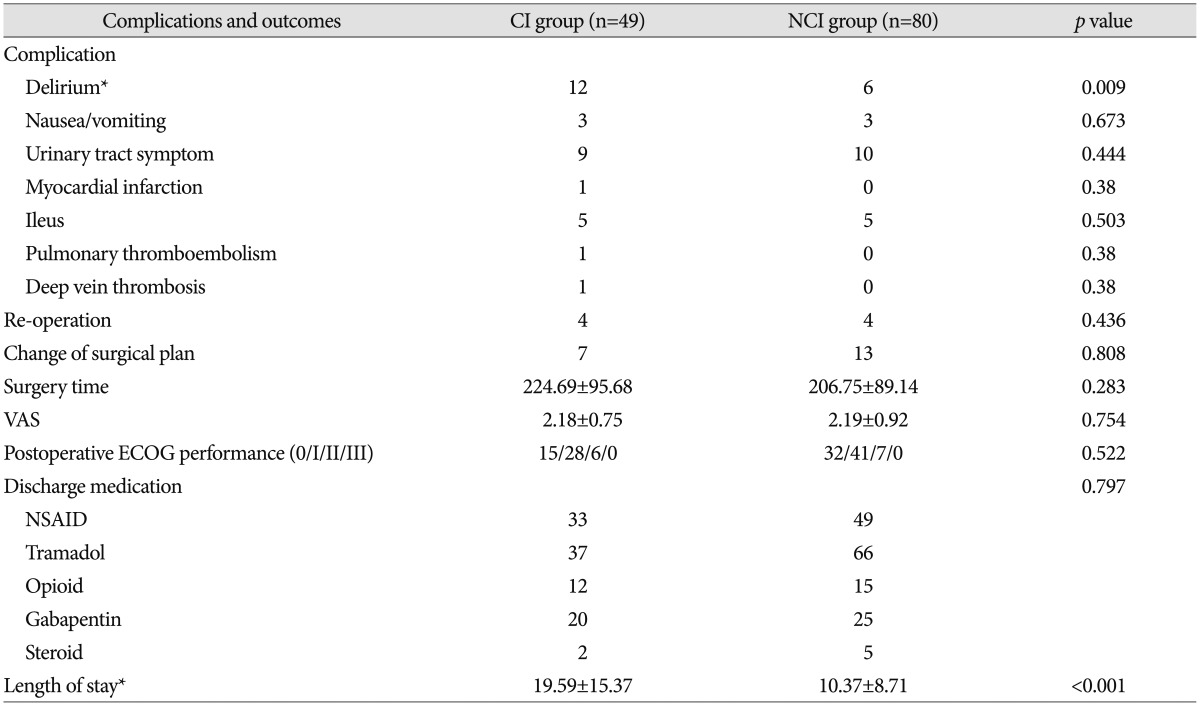
For the postoperative outcomes, we investigated the complications that occurred after the surgery. There were cases of delirium, nausea/vomiting, urinary tract symptoms, myocardial infarction, ileus, pulmonary thromboembolism, and deep vein thrombosis, but only delirium showed a significant difference between the two groups. Delirium showed a prevalence of 24% (12 of 49 patients) in the CI group and only 8% (6 out of 80 patients) in the NCI group (p=0.009). Four patients each in the CI group and NCI group underwent re-operation, and there was no statistically significant difference. There were 7 patients in the CI group and 13 patients in the NCI group who had changes in their surgical plans, but this was not a statistically significant difference. No significant differences were found between the two groups for surgery duration, pain after surgery, medication on discharge, and preoperative ECOG performance status (p>0.05). We investigated the length of stay for the CI group (19.59±15.37 days) and NCI group (10.37±8.71 days) and found a statistically significant difference and (p<0.001) (Table 3).
Inouye et al.67) have presented a multifactorial model for delirium, which showed a close association of postoperative delirium with predisposing factors (e.g., dementia and severe illness) and precipitating events (e.g., major surgery, anesthesia, and multiple psychoactive medications). The factor with the biggest impact on postoperative delirium in hip fracture and spine surgery has been reported to be preoperative dementia812). Although there have been reports about the precise assessment and incidence of preoperative dementia in cardiac surgery, few studies have been performed on the assessment and incidence of preoperative CI for spine surgery, in which subjective symptoms are very important.
CIs in elderly patients before and after surgery have mostly been studied for cardiac surgery. In coronary artery bypass surgery, PreCI prevalence has been reported as 35–45%514). Moreover, PreCI has been reported to be present in 20% of patients undergoing total hip joint replacements1). In our case, even after excluding the patients who had been diagnosed with dementia, 38% of the patients showed non-diagnosed CI, and, of these, 18% showed severe CI. These figures were similar to those reported for the cardiac surgery patients, which we suggest is probably because, like cardiac surgery, spine surgery patients are mostly elderly. Moreover, given that this level of prevalence before surgery is lower than that for hypertension but higher than those for diabetes mellitus or smoking, we suggest that there needs to be a preoperative assessment of CI. In addition, in order to investigate postoperative cognitive changes, baseline cognitive function needs to be accurately evaluated prior to surgery.
Partridge et al.11) have researched undiagnosed CI in older vascular surgical patients. In that study, CI or dementia was found in 68% of the patients (77 of 114), and it was previously unrecognized in 88.3% of the patients (68 of 77), which indicated that 60.5% of the patients (68 of 114) who were aged 60 years or older and who were presenting for vascular surgery had previously undiagnosed CI. Although these figures are higher than those of our study, a high number of patients with undiagnosed CI has been reported in several studies for vascular surgery and spine surgery.
Several studies have reported that postoperative delirium increases hospitalization, in-hospital cost, and mortality, and the reported risk factors include advanced age and a personal history of alcohol/drug abuse, depression, psychotic disorders, neurological disorders, anemia, fluid/electrolyte disorders, and weight loss. Moreover, postoperative delirium occurs more frequently in the PreCI group, and their duration of stay is longer2312). Therefore, interventions that increase the early detection of delirium have the potential to decrease the severity and duration of delirium and prevent unnecessary suffering and costs from the complications of delirium and unnecessary readmissions to the hospital2).
One limitation of this study was that only the MMSE was used to assess CI. Because there was a difference in educational background between the groups before surgery, additional assessments of CI are required, including a neurocognitive battery, a memory clinic diagnosis of vascular CI, and radiological study. Although the preoperative cognitive assessment was performed without interruption prior to the surgery, the physical environment or the patient's education level is likely to have affected the CI test results. Although we tried to select a homogenous group of patients who were undergoing lumbar spinal fusion at 65 years or older, the number of patients who were examined in this study was small, and there was no assessment of the surgery duration or anesthesia methods for lumbar spinal fusion. In the future, a large-scale assessment of preoperative CI and postoperative delirium in spinal surgery will be required.
A high prevalence of undiagnosed CI was discovered among elderly patients undergoing spine surgery. The existence of CI is associated with higher rates of postoperative delirium and prolonged hospital stays, which appears to affect clinical outcomes. Therefore, these results suggest that CI should be included in the preoperative evaluations that are performed prior to spine surgery on elderly patients.
References
1. Evered LA, Silbert BS, Scott DA, Maruff P, Ames D, Choong PF. Preexisting cognitive impairment and mild cognitive impairment in subjects presenting for total hip joint replacement. Anesthesiology. 2011; 114:1297–1304. PMID: 21502855.

2. Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013; 8:500–505. PMID: 23955965.

3. Fineberg SJ, Nandyala SV, Marquez-Lara A, Oglesby M, Patel AA, Singh K. Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine (Phila Pa 1976). 2013; 38:1790–1796. PMID: 23797502.

4. Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans : demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008; 47:302–310. PMID: 17936377.

5. Hogue CW Jr, Hershey T, Dixon D, Fucetola R, Nassief A, Freedland KE, et al. Preexisting cognitive impairment in women before cardiac surgery and its relationship with C-reactive protein concentrations. Anesth Analg. 2006; 102:1602–1608. PMID: 16717295.

6. Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999; 10:393–400. PMID: 10473946.

7. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996; 275:852–857. PMID: 8596223.

8. Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011; 59:2306–2313. PMID: 22188077.

9. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998; 351:857–861. PMID: 9525362.

10. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008; 108:18–30. PMID: 18156878.

11. Partridge JS, Dhesi JK, Cross JD, Lo JW, Taylor PR, Bell R, et al. The prevalence and impact of undiagnosed cognitive impairment in older vascular surgical patients. J Vasc Surg. 2014; 60:1002–1011.e3. PMID: 25017513.

12. Seo JS, Park SW, Lee YS, Chung C, Kim YB. Risk factors for delirium after spine surgery in elderly patients. J Korean Neurosurg Soc. 2014; 56:28–33. PMID: 25289122.

13. Shi Q, Warren L, Saposnik G, Macdermid JC. Confusion assessment method : a systematic review and meta-analysis of diagnostic accuracy. Neuropsychiatr Dis Treat. 2013; 9:1359–1370. PMID: 24092976.
14. Silbert BS, Scott DA, Evered LA, Lewis MS, Maruff PT. Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg. 2007; 104:1023–1028. PMID: 17456647.

15. Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer. 1992; 28A:1328–1330. PMID: 1515244.

16. Xu T, Bo L, Wang J, Zhao Z, Xu Z, Deng X, et al. Risk factors for early postoperative cognitive dysfunction after non-coronary bypass surgery in Chinese population. J Cardiothorac Surg. 2013; 8:204. PMID: 24175992.

Table 2
Patient characteristics

CI : cognitive impairment, NCI : non-cognitive impairment, ECOD : Eastern Cooperative Oncology Group, NSAID : nonsteroidal anti-inflammatory drugs, PHLD : partial hemilaminectomy and discectomy, PSF : pedicle screw fixation, TLIF : transforaminal lumbar interbody fusion, DLIF : direct lumbar interbody fusion, OLIF : oblique lumbar interbody fusion, BUN : blood urea nitrogen, WBC : white blood cell




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download