Abstract
Organized hematoma is a rare complication that can develop following gamma knife radiosurgery (GKS) for cerebral arteriovenous malformation (AVM). Here, we describe 5 patients with growing organized hematomas that developed from completely obliterated AVMs several years after GKS. The patients were 15, 16, 30, 36, and 38 years old at the time of GKS, respectively, and 3 patients were female. Four AVMs were located in the lobe of the brain, and the remaining AVM were in the thalamus. Between 2-12 years after GKS, patients developed progressive symptoms such intractable headache or hemiparesis and enhancing mass lesions were identified. Follow-up visits revealed the slow expansion of the hematomas and surrounding edema. Steroids were ineffective, and thus surgery was performed. Histology revealed organized hematomas with a capsule, but there was no evidence of residual AVMs or vascular malformation. After surgery, the neurological symptoms of all patients improved and the surrounding edema resolved. However, the hematoma continued to expand and intraventricular hemorrhage developed in 1 patient whose hematoma was only partially removed. GKS for cerebral AVM can be complicated by growing, organized hematomas that develop after complete obliteration. Growing hematomas should be surgically evacuated if they are symptomatic. Radical resection of the hematoma capsule is also strongly recommended.
Gamma knife radiosurgery (GKS) has become an effective and safe treatment for cerebral arteriovenous malformation (AVM) in recent years. Most complications that develop after GKS are due to the radiation-induced necrosis and hemorrhage of treated but not yet obliterated AVMs. However, some late complications, such as radiation-induced tumor5), cavernous malformation917), cyst formation1414), and expanded hematoma, may develop several years after GKS, even if the AVMs have been completely obliterated. These complications can cause mass effects and, if symptomatic, are very important clinically. In some cases, these complications require surgical intervention. Here, we report 5 cases of delayed, expanding, organized hematomas that developed in patients with cerebral AVMs that had been completely obliterated using GKS. The hematomas were treated by surgical evacuation.
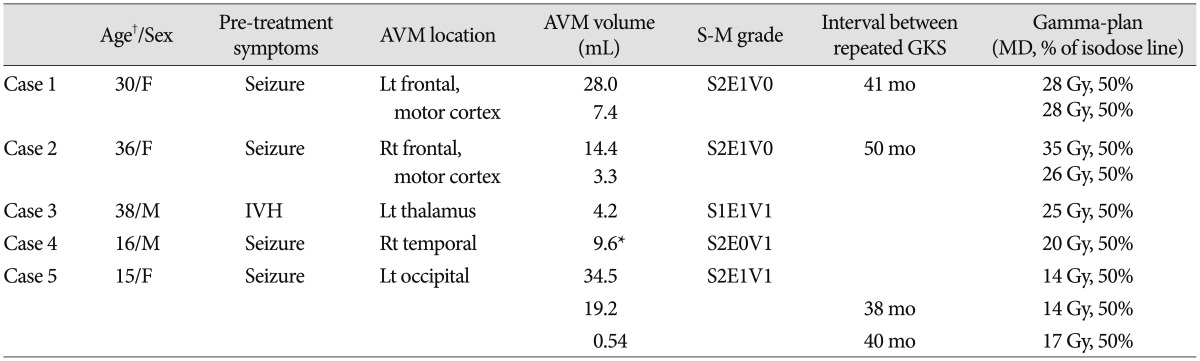
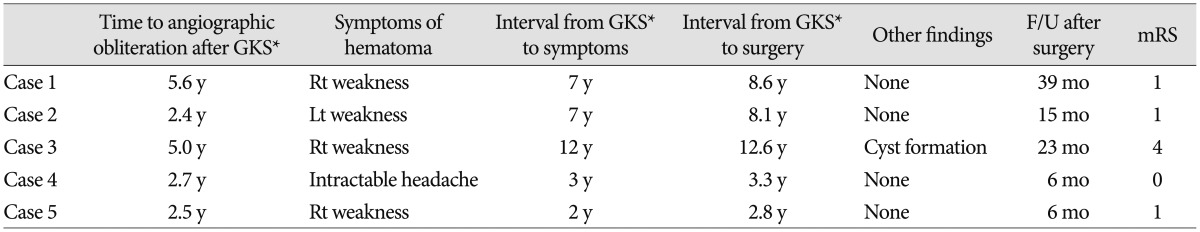
We retrospectively reviewed 2 male and 3 female patients who were between 15-38 years of age at the time of GKS. The characteristics of these patients are summarized in Table 1. Because 3 patients (case 1, 2, and 5) received GKS at another hospital and were referred for follow-up examinations, the precise rate of developing expanding hematomas remains unclear. These 3 patients underwent second GKS for remnant AVM at 38, 41, and 50 months after the first GKS, respectively. One patient (case 5) underwent a third GKS at 40 months after the second GKS. The initial presenting symptoms included seizure in 4 patients and intraventricular hemorrhage in 1 patient (case 3). Although there was some diversity, 4 AVMs were classified as Spetzler-Martin grade 3 and 1 AVM was classified as grade 4. Nidus volume at the time of the first GKS ranged between 4.2-34.5 mL. One patient (case 4) underwent partial embolization with Onyx prior to GKS. Fourteen to 35 Gy of radiation was delivered to the margin of the AVM, achieving an isodose level of 50%.
The GKS treatment results are summarized in Table 2. Cerebral angiography was performed between 29-67 months after the last GKS and confirmed the complete obliteration of the nidi. Serial magnetic resonance imaging (MRI) was used to evaluate all patients. In 4 patients (case 1, 2, 4, and 5), changed in radiation-induced imaging were observed 1-2 years after GKS; however all patients were asymptomatic and mild changes in imaging did not indicate mass effects on the surrounding edema. Cyst formation was detected 6 years after GKS in the remaining patient (case 3); an Ommaya reservoir was subsequently placed in this patient.
New enhancing mass lesions were detected 2-12 years after the last GKS. These lesions were associated with the surrounding brain edema and were symptomatic in all patients; 4 patients had progressive hemiparesis, and 1 patient had intractable headache (case 4). These symptoms did not improve when steroids were administered, and the enhancing lesions progressively increased in size on serial follow-up examinations. Our impression was that these lesions were radiation-induced necroses or cavernous malformations that developed after GKS.
All five study patients underwent a craniotomy, and the mass lesions were removed. Histological findings revealed that all lesions were organized hematomas with a capsule; there was no evidence of cavernous malformation. The neurological symptoms in 3 of 4 patients improved after surgical removal, and the surrounding brain edema was largely resolved. Modified Rankin scale (mRS) scores were 0 or 1. However, 1 patient, whose hematoma had only been partially removed (case 3), demonstrated transient neurological and radiological improvements that were then followed by symptom aggravation and recurrent bleeding. His final mRS score was 4 at 2 years after the onset of symptom aggravation.
A 36-year-old female patient underwent GKS for right motor cortex AVM (13.4 mL volume) and received a marginal dose of 35 Gy in August 1998. Fifty months after the first GKS, she underwent a second GKS for the remaining AVM (3.3 mL volume) (Fig. 1A, B), in which a marginal dose of 26 Gy was administered. MRI performed 18 months after the second GKS revealed mild, asymptomatic, radiation-induced changes. Cerebral angiography was performed 29 months after the second GKS, which revealed the complete obliteration of the nidus (Fig. 1C). Seven years after the second GKS, the patient was transferred to our institution because of progressive left hemiparesis. Brain MRI revealed a gadolinium-enhanced hemorrhagic mass measuring 4×3.5×3 cm that was surrounded by extensive edema (Fig. 1D). However, cerebral angiography was performed around the same time, which showed the complete obliteration of the AVM. Brain MRI performed 7 months later revealed that the lesion had expanded, and the surrounding edema had become increasingly marked (Fig. 1E).
Symptoms did not improve following the administration of steroid. Therefore, microsurgery was performed on the expanding mass lesion at 97 months after the second GKS. This was identified as a hypovascular, yellowish, and rubbery mass with a tough capsule that contained multiple layers of organized hematoma. The entire mass and capsule were surgically removed. Brain edema and neurological symptoms and signs disappeared afterward. Postoperative MRI revealed significant improvement in mass effects (Fig. 1F).
Histological examination of the resected tissues revealed obliterated AVM vessels and an organizing hematoma (Fig. 2A). The hematoma contained organized, well-capsulated, multi-stage clots with hemosiderin deposits and thrombus (Fig. 2B, C). No indications of cavernous malformation were found.
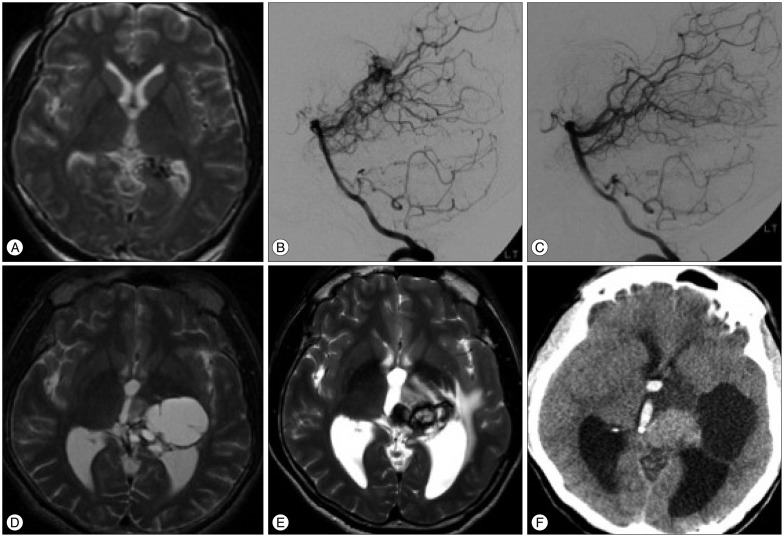
A 38-year-old male patient underwent GKS because of an AVM located in the left thalamus and posterior hippocampal gyrus (4.2 mL volume) (Fig. 3A, B), and a marginal dose of 25 Gy was administered. Cerebral angiography performed 60 months after GKS confirmed the complete obliteration of the AVM (Fig. 3C). A cyst at the previous AVM site developed 6 years after GKS (Fig. 3D), and the placement of an Ommaya reservoir was necessary. Follow-up images revealed that the cyst has not increased in size. However, an enhancing mass lesion associated with the cyst developed 12 years after GKS (Fig. 3E). The patient complained of left hemiparesis and severe headache.
Symptoms did not improve after administering steroids, and the patient underwent left parieto-occipital craniotomy. The operative field was limited due to the deep location of the lesion and the surrounding hypothalamus and midbrain. The lesion was hypovascular, gray, firm, and had a tough capsule. The mass and the capsule were removed along with the residual hematoma. Computed tomography (CT) was performed during the immediate postoperative period, which revealed the incomplete resection of the hematoma. Histological examination revealed an organized hematoma without any indications of cavernous malformation.
After surgery, the perilesional edema and neurological symptoms of the patient improved. However, 11 months after surgery, he developed a sudden loss of consciousness and right hemiplegia. Brain CT revealed intracerebral and intraventricular hemorrhage with hydrocephalus (Fig. 3F). The patient underwent external ventricular drainage and received conservative management thereafter. After 2 years of clinical follow-up examinations, the mRS score of the patient was 4, which indicates moderately severe disability.
We here describe an unusual type of hematoma that developed at the site of a completely obliterated AVM several years after GKS in 5 patients. While a chronic, organized, growing hematoma is a rare complication that develops after radiosurgery for AVM, there are several cases in the literature that describe this hematoma as growing from residual AVM681415) or a totally obliterated AVM71016) following radiosurgery. In our cases described here, chronic, organized, growing hematomas developed despite cerebral angiographic confirmation of total AVM obliteration and were further characterized by their late onset, slow expansion, and capsule formation. While previous histological examinations69) of expanding hematomas that developed after GKS report cavernous malformations (with or without radiation necrosis), the histological findings of our current series only revealed organized hematoma and a tough collagenous capsule; radiation necrosis and de novo cavernous malformation were not observed. Growing hematomas in association with cysts have also been reported13), as noted in 1 patient (case 3) in our current series.
The initial source of a chronic encapsulated intracerebral hematoma is often a vascular anomaly69). A number of possibilities have been proposed. Roda et al.12) proposed that chronic organized hematomas are probably caused by small vascular malformations that are destroyed or thrombosed during hemorrhagic episodes. Pozzati et al.11) suggested that such hematomas are due to occult, self-destroying vascular malformations, such as cavernous angioma or venous angioma. Hirsh et al.3) proposed that the hematoma capsule is composed of fibroblasts derived from the abnormal vessels of occult vascular malformations. However, how chronic organized hematomas develop from obliterated AVMs following GKS remains unclear. Lee et al.7) have suggested that the most likely initial cause is minor recurrent bleeding from fragile vessels within radionecrotic brain tissue. Moreover, like chronic subdural hematoma, the encapsulated wall of a chronic organized hematoma may promote the gradual growth of these hematomas, which occurs because of recurrent bleeding or exudation from the capillaries in the inner layer of the capsule10). It is also possible that AVM degeneration after radiosurgery triggers the formation of organized hematomas16). Moreover, Nakamizo et al.10) reported that the vascular endothelial growth factor (VEGF) pathway is activated after radiosurgery for AVM, which indicates increased angiogenesis; this could lead to chronic organizing hematoma. Only 1 of our current patients initially presented with hemorrhage and, during the latent period, no additional bleeding from the AVM was noted. However, in 3 of our present cases, mild radiation-induced changes in imaging were observed 1 or 2 years after GKS, although these changes were symptomless, and another patient developed cysts 6 years after GKS. Our histological examinations suggest that the hemorrhage was due to recurrent bleeding from the fragile vessels contained within the thick hematoma capsule, and there was no evidence of associated vascular anomalies. Although it cannot be excluded that the initial bleeding episode initiated the hematomas in our present cases, our observations suggest that continuous minor bleeding from necrotic brain tissue results in the expansion of hematomas.
Radiation-induced changes on MRI including perilesional edema are a significant clinical problem. In these situation, symptomatic patients are generally treated using corticosteroid therapy218). Our experience suggests that chronic organized hematomas associated with GKS for AVM grow gradually and become symptomatic when there is severe edema in the surrounding brain structure. Surgical removal is necessary, if the hematoma and perilesional edema increase in size and become symptomatic, especially because steroid therapy does not effectively control the edema associated with the expanding hematoma and because this rare condition is progressive. After the expanding hematoma is removed, the peripheral brain edema will likely decrease and the neurological symptoms will probably immediately improve. However, the whole capsule should be removed : partial resection does not efficiently control this growing hematoma.
The risk factors for developing chronic organized hematoma remain unknown. When Takeuchi et al. summarized the clinicoradiological features of 7 patients with growing hematomas that developed after radiosurgery15), they identified male sex, high frequency of basal ganglia involvement, long interval from radiosurgery to diagnosable hematoma, and high rate of residual nidus as associating factors. In contrast, in our 5 cases described here, all growing hematomas arose from completely obliterated AVMs (albeit after several years) and the AVMs were mainly located in superficial areas (4 AVM were located in the lobes, while 1 patient had an AVM in the thalamus). Some predisposing factors were identified in our current patients, although the actual rate and risk factors for growing hematoma after radiosurgery remain undetermined due to the low incidence of complications. We propose large volume AVM (longest diameter >3 cm in 4 cases), repeated radiosurgery, and large cumulative radiation dose as predisposing factors. Further studies are needed to ascertain the risk factors and mechanisms of growing hematomas that developed following GKS for AVM.
Chronic organized hematoma is a late complication that develops after GKS to treat AVM, even after complete obliteration. Although this is a rare complication, chronic organized hematomas seem to develop in patients with a high volume of AVMs who receive repeated radiosurgeries and high cumulative dose of radiation. To manage these symptoms, a chronic organized hematoma that surrounds the radiated AVM should be completely surgically resected. This will cause the surrounding brain edema to rapidly decrease and quickly resolve neurological symptoms.
References
1. Al Hinai Q, Tampieri D, Souhami L, Sadikot A, Sinclair D, Leblanc R. Cyst formation following radiosurgery for AVMs : report of 3 cases. Can J Neurol Sci. 2011; 38:734–740. PMID: 21856577.
2. Clark AJ, Butowski NA, Chang SM, Prados MD, Clarke J, Polley MY, et al. Impact of bevacizumab chemotherapy on craniotomy wound healing. J Neurosurg. 2011; 114:1609–1616. PMID: 21142749.

3. Hirsh LF, Spector HB, Bogdanoff BM. Chronic encapsulated intracerebral hematoma. Neurosurgery. 1981; 9:169–172. PMID: 7266817.

4. Izawa M, Chernov M, Hayashi M, Nakaya K, Kamikawa S, Kato K, et al. Management and prognosis of cysts developed on long-term follow-up after Gamma Knife radiosurgery for intracranial arteriovenous malformations. Surg Neurol. 2007; 68:400–406. discussion 406PMID: 17905064.

5. Kaido T, Hoshida T, Uranishi R, Akita N, Kotani A, Nishi N, et al. Radiosurgery-induced brain tumor. Case report. J Neurosurg. 2001; 95:710–713. PMID: 11596968.
6. Kurita H, Sasaki T, Kawamoto S, Taniguchi M, Kitanaka C, Nakaguchi H, et al. Chronic encapsulated expanding hematoma in association with gamma knife stereotactic radiosurgery for a cerebral arteriovenous malformation. Case report. J Neurosurg. 1996; 84:874–878. PMID: 8622164.

7. Lee CC, Pan DH, Ho DM, Wu HM, Chung WY, Liu KD, et al. Chronic encapsulated expanding hematoma after gamma knife stereotactic radiosurgery for cerebral arteriovenous malformation. Clin Neurol Neurosurg. 2011; 113:668–671. PMID: 21507569.

8. Maruyama K, Shin M, Tago M, Kurita H, Kawahara N, Morita A, et al. Management and outcome of hemorrhage after Gamma Knife surgery for arteriovenous malformations of the brain. J Neurosurg. 2006; 105(Suppl):52–57. PMID: 18503330.

9. Motegi H, Kuroda S, Ishii N, Aoyama H, Terae S, Shirato H, et al. De novo formation of cavernoma after radiosurgery for adult cerebral arteriovenous malformation--case report. Neurol Med Chir (Tokyo). 2008; 48:397–400. PMID: 18812682.

10. Nakamizo A, Suzuki SO, Saito N, Shono T, Matsumoto K, Onaka S, et al. Clinicopathological study on chronic encapsulated expanding hematoma associated with incompletely obliterated AVM after stereotactic radiosurgery. Acta Neurochir (Wien). 2011; 153:883–893. PMID: 20931239.

11. Pozzati E, Giuliani G, Gaist G, Piazza G, Vergoni G. Chronic expanding intracerebral hematoma. J Neurosurg. 1986; 65:611–614. PMID: 3772447.

12. Roda JM, Carceller F, Pérez-Higueras A, Morales C. Encapsulated intracerebral hematomas : a defined entity. Case report. J Neurosurg. 1993; 78:829–833. PMID: 8468616.
13. Shuto T, Matsunaga S, Suenaga J. Surgical treatment for late complications following gamma knife surgery for arteriovenous malformations. Stereotact Funct Neurosurg. 2011; 89:96–102. PMID: 21293169.

14. Shuto T, Ohtake M, Matsunaga S. Proposed mechanism for cyst formation and enlargement following Gamma Knife Surgery for arteriovenous malformations. J Neurosurg. 2012; 117(Suppl):135–143. PMID: 23205801.

15. Takeuchi S, Takasato Y, Masaoka H. Chronic encapsulated intracerebral hematoma formation after radiosurgery for cerebral arteriovenous malformation. Neurol India. 2011; 59:624–626. PMID: 21891948.

16. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, et al. Development of chronic encapsulated intracerebral hematoma after radiosurgery for a cerebral arteriovenous malformation. Acta Neurochir (Wien). 2009; 151:1513–1515. PMID: 19597762.

17. Wang X, Hui XH, Liu JP, Mao Q. Radiation-induced cavernous malformation at the site of arteriovenous malformation following gamma knife radiosurgery : case report. Clin Neurol Neurosurg. 2012; 114:1287–1289. PMID: 22456056.

18. Williams BJ, Park DM, Sheehan JP. Bevacizumab used for the treatment of severe, refractory perilesional edema due to an arteriovenous malformation treated with stereotactic radiosurgery. J Neurosurg. 2012; 116:972–977. PMID: 22324417.

Fig. 1
Images obtained during the clinical course of patient 2. A and B : Axial T2-weighted image and right internal carotid angiograms showing a residual arteriovenous malformation (AVM) in the right frontal lobe at 4 years after the first gamma knife surgery (GKS). C : Right internal carotid angiograms obtained 29 months after the second GKS. Residual AVM was not observed. D : T2-weighted magnetic resonance image (MRI) obtained 7 years after the second GKS and after the patient developed hemiparesis. Edema surrounding the obliterated AVM is shown. E : T2-weighted MRI obtained 7 months after the preceding MRI revealed that the heterogenous signal mass lesion had expanded and the edema was even more intense. F : T2-weighted MRI obtained 2 months after surgical excision. The mass lesion was not observed and the mass effect improved.
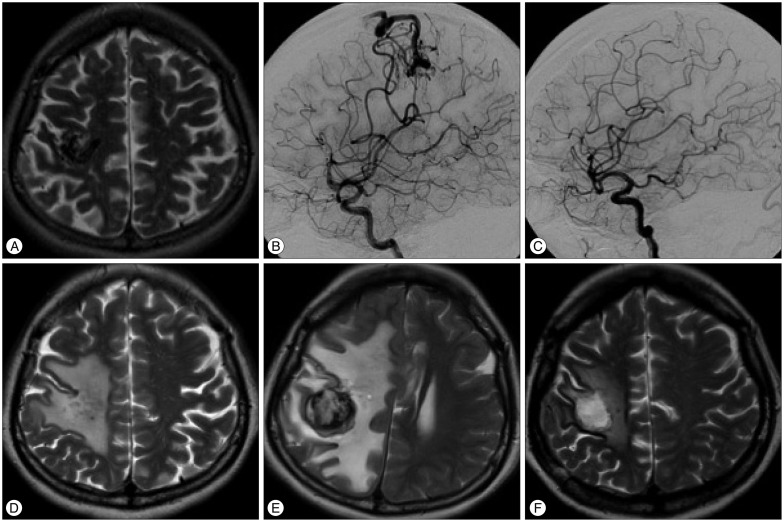
Fig. 2
Histological results for the specimen resected from patient 2. A : Gross image showing that the tough capsule contained xanthochromic fluid, thrombosis, and fibrotic tissue. B : Hematoxylin and eosin-stained specimen. Multi-stage clots with hemosiderin deposits and fresh hemorrhage (×100 original magnification). C : Hematoxylin and eosin-stained specimen. The hematoma contained fibrotic and degenerated vessels (arrows), which are suggestive of obliterated arteriovenous malformation vessels (×12.5 original magnification).
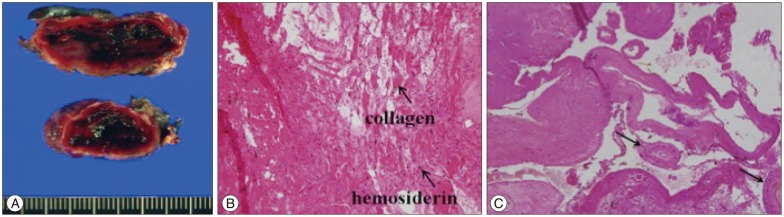
Fig. 3
Images obtained during the clinical course of patient 3. A and B : Axial T2-weighted image and left vertebral angiograms showing the presence of an arteriovenous malformation (AVM) in the left thalamus and posterior hippocampal gyrus. C : Vertebral angiogram obtained 5 years after gamma knife surgery (GKS) showing no residual AVM. D : A cyst developed 6 years after GKS and an Ommaya reservoir was inserted. E : T2-weighted magnetic resonance image (MRI) obtained 12 years after GKS when the patient developed left hemiparesis and severe headache, which shows a heterogenous signal mass and extensive surrounding edema without an enlarged cyst. F : Computed tomography performed 11 months after subtotal resection revealed that the hematoma had expanded again and confirmed the presence of intraventricular hemorrhage.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download