Abstract
Direct removal of beak-type ossification of posterior longitudinal ligament at thoracic spine (T-OPLL) is a challenging surgical technique due to the potential risk of neural injury. Slipping off the cutting surface of a high-speed drill may result in entrapment in neural structures, leading to serious complications. Removal of T-OPLL with an ultrasonic osteotome, utilizing back and forth micro-motion of a blade rather than rotatory-motion of drill, may reduce such complications. We have applied the ultrasonic osteotome for posterior circumferential decompression of T-OPLL for three consecutive patients with beak-type OPLL and have described the surgical techniques and patient outcomes. The preoperative chief complaint was gait disturbance in all patients. Japanese orthopedic association scores (JOA) was used for functional assessment. Scores measured 2/11, 5/11, 2/11, and 4/11 for each patient. The ventral T-OPLL mass was exposed after posterior midline approach, laminotomy and transeversectomy. The T-OPLL mass was directly removed with an ultrasonic osteotome and instrumented segmental fixation was performed. The surgeries were uneventful. Detailed surgical techniques were presented. Gait disturbance was improved in all patients. Dural tear occurred in one patient without squeal. Postoperative JOA was 6/11, 10/11, 8/11, and 8/11 (recovery rate; 44%, 83%, 67%, and 43%) respectively at 18, 18, 10, and 1 months postoperative. T-OPLL was completely removed in all patients as confirmed with computed tomography scan. We hope that surgical difficulties in direct removal of T-OPLL might be reduced by utilizing ultrasonic osteotome.
Thoracic ossification of posterior longitudinal ligament (T-OPLL) often requires surgical treatment in cases of symptomatic progressive myelopathy. There are various kinds of surgical options for thoracic OPLL. Direct decompression could be achieved with anterior, posterior or a combined approach. Indirect decompression could be achieved with laminectomy, laminoplasty or posterior instrumented correction of thoracic kyphosis5811121314151826272830). Although direct removal of T-OPLL is ideal for neural element decompression, complications associated with the anterior approach, e.g., neurologic deficit and dural tear/cerebrospinal fluid (CSF) leakage, leads us to explore other potential surgical options513141718). Because laminectomy had a poor outcome due to progression of thoracic kyphosis and persistent ventral compression, instrumented fusion with or without direct removal of T-OPLL mass with posterior approach was introduced with comparable recovery rate to anterior approach59111213141819). Although indirect decompression can be achieved with this technique13), circumferential decompression is sometimes a more suitable surgical option8111227). However, in these instances, a complication rate of approximately 30% has been encountered, most notably in beak-type T-OLL (growth of OPLL mass across a disc space at a single segment)131528). The OPLL mass is traditionally removed with a high-speed drill581011122130). Slipping off the cutting surface and entrapping important soft tissues, such as neural structure, may leads to serious complication. The ultrasonic osteotome is a recent introduction to the clinical field27202324). Such devices utilize back and forth micro-motion rather than rotatory-motion to allow precise removal of bone with minimal impact to the adjacent soft tissues27202324). Energy from the cutting edges of these devises is preferentially transmitted to hard structures (bone)27202324). Adjacent soft tissues (ligamentum flavum, posterior longitudinal ligament, dura) are spared as these structures can bend, vibrate and deform to vibratory micromotion27202324). The advantage of a tissue selective cutting device may be utilized in the removal of compressive T-OPLL. We have applied the ultrasonic osteotome for posterior circumferential decompression of T-OPLL in a series of patients and have described the surgical technique and patient outcomes.
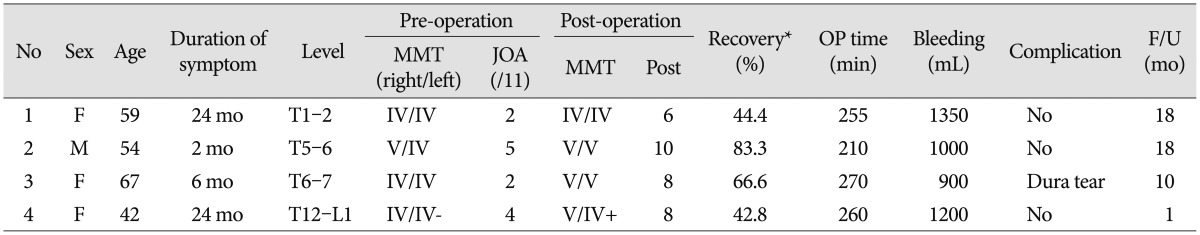
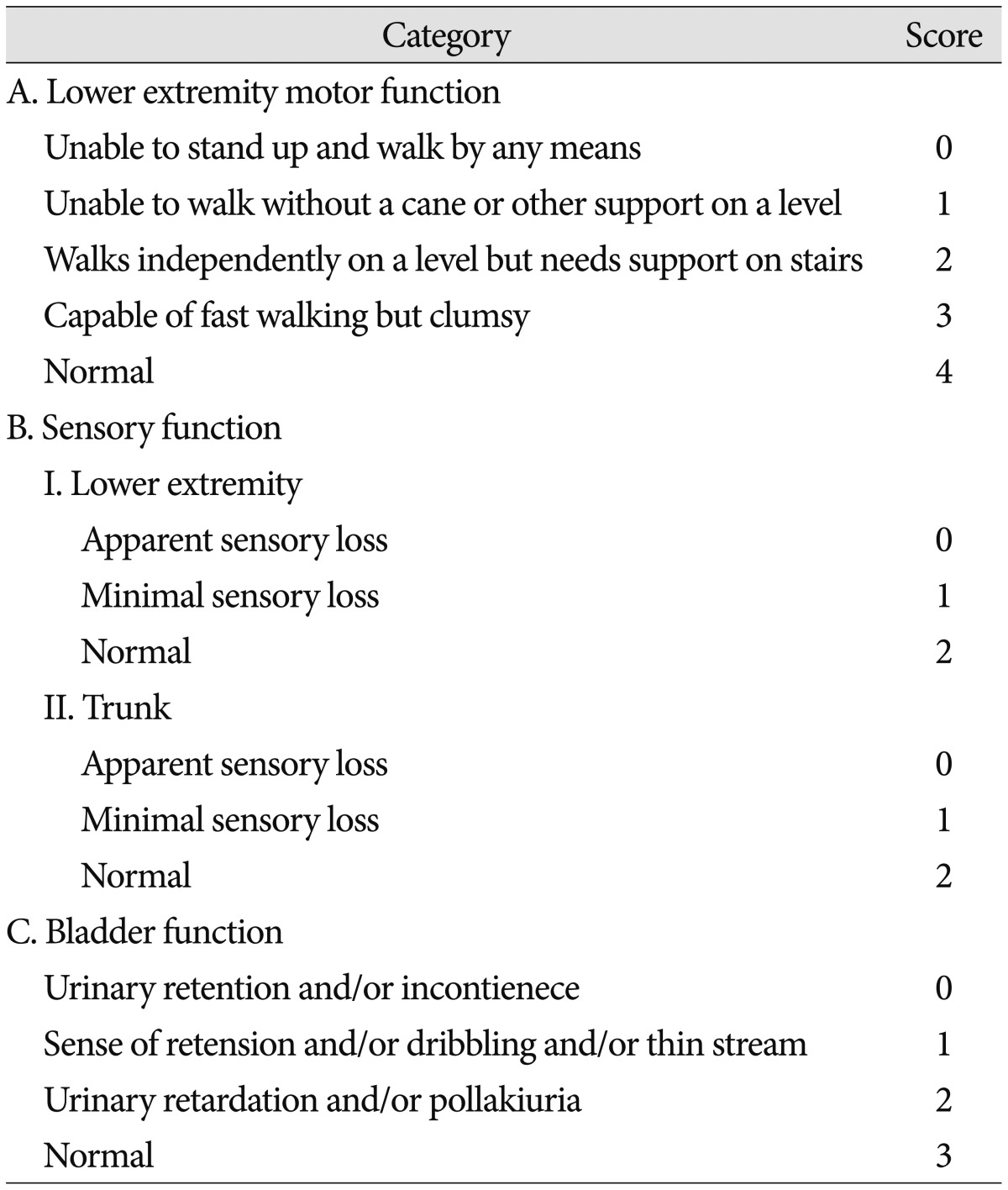
Starting October 2012, we have applied the ultrasonic osteotome for four consecutive patients with beak-type OPLL (Table 1). The preoperative chief complaint was gait disturbance in all patients. Two patients underwent cervical laminoplasty 2 and 3 years previously secondary to cervical OPLL (Case 1 and 3). Their main problem was paraparesis and symptom was partially improved in Case 1 and normalized in Case 3 after cervical laminoplasty. However, gait disturbance was aggravated 12 months and 30 months after initial surgery, respectively. In the other patients, there was no history of spine surgery. Neurological status was assessed with the Japanese Orthopedic Association (JOA) scoring system for thoracic myelopathy (Table 2)811121314). Magnetic resonance (MR) imaging and/or computed tomography (CT) scan were obtained both pre- and post-operatively and patients were scheduled to be followed-up at postoperative month 1, 3, 6, 12 and yearly thereafter.
Posterior circumferential decompression and instrumented fusion was performed in all patients. Intraoperative neuromonitoring [transcranial motor evoked potential (TcMEP) and somatosensory evoked potential (SSEP)] were applied in all patients, but TcMEP was not obtainable in 2 patients (Case 1 and 3). All operations were performed via midline skin incision. First, pedicle screws were inserted 1-2 levels above and 1-2 levels below the index level. The lateral margin of lamina was cut at the junction of facet joint with the ultrasonic osteotome (BoneScalpel, Misonix, NY, USA) (Fig. 1). The lamina was gradually cut with ultrasonic osteotome by slight pushing down its blade against lamina with a vertical motion. When inner cortical bone was cut, loss of resistance was felt and the ostetome was not inserted beyond the depth (Fig. 2A) (Supplementary Video 1 in the online-only Data Supplement). After elevation of cut lamina with clamp, the cranial and caudal ligamentum flavum of elevated lamina was resected with Kerrison rongeur. After laminotomy, the remained facet joint, transverse process and pedicles were removed with conventional rongeur and punch. Spinal nerve roots at the index level were resected bilaterally after no change in intraoperative neuromonitoring (INM) upon 10 minutes of neurovascular bundle clipping. After dissecting the lateral margin of the OPLL mass from the dura, the superior and inferior margin of the beak-type OPLL mass was identified. The base of OPLL mass was removed initially. Next, the OPLL mass attached to dura was removed with the ultrasonic osteotome (Fig. 2B). Use of the ultrasomic osteotome allowed direct resection of the OPLL mass under gentle dural retraction with a suction tip until half of its width was resected. A similar technique was repeated on the contralateral side. After removal of OPLL mass, the removed lamina was replaced by connecting to cranial and caudal lamina with miniplate or translaminar screw (Fig. 3, 4)2229). Finally, rods were fixed to pedicle screws and pedicle screws were inserted at the index vertebra to enhance stability and reduce extent of surgery1). After grafting allobone chips on the decorticated lamina, the surgical wound was closed in a layer by layer fashion.
Patient characteristics, preoperative and postoperative neurological status are described in Table 1. There was no change in TcMEP in Case 2 and 4, and no change in SSEP in all cases. Dura tear was occurred in one case and it was repaired with adhesive sealant without neurological sequel. Postoperative neurological deficits were not encountered in any patients. All patients were discharged within 1 week after surgery. Gait disturbance was improved in Case 2, 3 and 4, but independent walking without assistance was not recovered in Case 1.
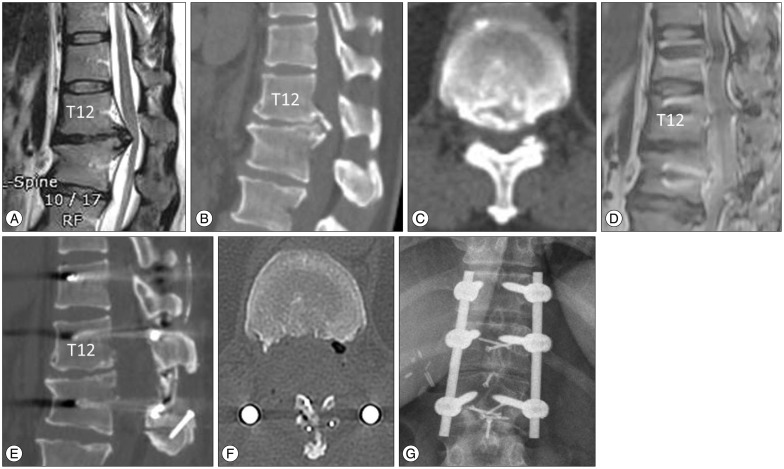
A 67-year-old female patient presented with 6 months of paraparesis. Preoperative MR imaging and CT scan showed a beak-type OPLL mass and spinal cord compression at T5-6 (Fig. 3A-D). Cervical laminoplasty was performed 3 years prior due to cervical OPLL, but paraparesis (motor power grade IV/V) developed 2.5 years after her previous surgery. Preoperative JOA score was 2/11 (1+0+0+1). Posterior circumferential decompression and instrumented fusion was performed with the use of the ultrasonic osteotome. With the help of tissue selectivity of ultrasonic osteotome, the OPLL mass could be directly resected from the dura. The final portion of OPLL was difficult to remove, once the mass lost connection with the base of the OPLL mass, due to ineffective transmission of ultrasonic energy. During the operation, there was no significant change in intraoperative SSEP monitoring. TcMEP was not detectable. Dural injury was occurred during removal of OPLL and the defect was repaired with adhesive dural sealant. Postoperative CT scan showed nearly complete removal of the OPLL mass (Fig. 3E-H), but a small fragment of OPLL mass was left (Fig. 2G). The lamina was replaced with mini-plates (Fig. 3I). There was no sequelae associated with the dural tear. The patient's preoperative paraparesis was improved and independent ambulation was possible 1 month after surgery. Ten months after the date of surgery, the JOA score was 8/11 (2+2+2+2); a recovery rate of 67% (Supplementary Video 2 in the online-only Data Supplement).
Different from Case 3, the base of OPLL mass was removed with ultrasonic osteotome and the OPLL mass was detached and removed from the dura. Because the energy was emitted from the end of osteotome, the T12 root could be safely retracted with the osteotome during resection of OPLL (Fig. 2B), and the T12 root was preserved. Preoperative paraparesis was improved at postoperative month 1 (a recovery rate of 43%) (Supplementary Video 3 in the online-only Data Supplement).
In the present cases report we have shown the feasibility of utilizing the ultrasonic osteotome for posterior circumferential decompression operations for thoracic beak-type OPLL. The ultrasonic osteotome has the advantage of tissue selectivity and eliminates the dangerous rotatory motion of the high speed burr, thus allowing a more safe removal of T-OPLL. The nerve root could be preserved with this technique, because of non-rotatory motion of ultrasonic osteotome.
There are various kinds of surgical options for beak-type T-OPLL. Direct decompression could be obtained with anterior, posterior or a combined approach. Indirect decompression could be achieved with laminectomy, laminoplasty or a posterior instrumented correction of thoracic kyphosis5811121314151826272830).
Matsumoto et al.13) evaluated various surgical approaches for beak-type T-OPLL in 45 patients; 8 patients underwent laminectomy, 16 underwent laminoplasty, 12 underwent anterior decompression via anterior approach, 5 underwent anterior decompression via posterior approach, 2 underwent both anterior and posterior approach and 2 underwent anterior decompression via sternum splitting approach13). Clinical outcome, surgical technical difficulty and safety are important factors in determining the desired approach. Regarding clinical outcome, removal of the T-OPLL mass seems to be associated with a better outcome; a mean recovery rate of JOA was about 60% with circumferential decompression and about 30-40% with only posterior decompression1328). An anterior approach may be preferable to remove T-OPLL, but the transthoracic/extraplerual approach or trans-sternal approach is technically challenging and is associated with a higher complication rate41618). A recent review on this subject has shown that neurological deficit occurred in 2.7-18.8% of patients after an anterior approach51718). Direct removal of the ossified mass with a posterior only approach may be an alternative, but it has been traditionally challenging secondary to associated kyphotic deformities, adhesion of T-OPLL to the dura and a narrow/blind surgical field316). Direct removal of T-OPLL mass was associated with a high neurological complication rate; neurological deficit occurred when T-OPLL was excised in 7/48 (14.2%), thinned and floated in 7/25 (28%), and left untouched in 4/81 (4.9%)13). The rate of neurologic deficit was especially high for patients with beak-type T-OPLL (30%)11152628). Therefore indirect decompression through instrumented correction of thoracic kyphosis after posterior decompression was suggested as a safe alternative to allow for floating back of the spinal cord132830). However, postoperative magnetic resonance imaging (MRI) demonstrated that indirect decompression was not sufficient after correction of kyphosis alone in 13 out of 15 patients, and 11 patients underwent a secondary procedure for anterior decompression8). Matsuyama et al.16) showed that neurological deterioration occurred in 5/21 patients with beak-type T-OPLL after posterior indirect decompression because of persistent ventral compression. In addition, growth of beak-type T-OPLL was observed 3 years after laminectomy and corrected fusion of thoracic kyphosis25). Although indirect decompression through instrumented correction of kyphsosis was an alternative, considering those results together, direct removal of T-OPLL, especially for patients with beak-type T-OPLL, seems to be an appropriate surgical option.
Neurological deficits usually occurred immediately after surgery in cases of T-OPLL. Therefore, surgical insult to the neural tissue must not be disregarded111226). During surgery, the T-OPLL mass is traditionally removed with a high-speed drill and curette. Neurologic injury may occur during removal of T-OPLL just beneath dura with high-speed drill11). When there are strong adhesions of T-OPLL mass to the dura, use of high-speed drill for removal of T-OPLL may be dangerous secondary to rotatory-motion of the drill6). Ultrasonic osteotome has been used in various surgeries such as plastic surgery, but the usage was not widely spread in spine surgery267202324). Ultrasonic osteotome utilize back and forth micro-motion rather than rotatory-motion, thus energy from the cutting edges of these devises is preferentially transmitted to hard structures, such as lamina27202324). The dura can bend, vibrate and deform to vibratory micromotion with minimal transmission of energy27202324). Moreover, the safety of surgical procedure may be enhanced, because movement of its tip is limited to a fine vibration without problem of bounce of rotatory drill6). This features enable selective removal of beak type OPLL with minimal injury to the dura and spinal cord. The relative tissue selectivity allowed for brief contact with the dura, and thin shaving of T-OPLL and preservation of nerve root were possible (Fig. 2B)27202324). The ultrasonic osteotome may be preferable for removal of T-OPLL over a high-speed drill in this regards27202324). However, the laminotomy with ultrasonic osteotome is performed without direct visualization of dura, and spinal cord injury may occur by insertion of blade beyond the inner cortex of lamina, if penetration of lamina is not felt. Moreover, the pressure of irrigation fluid may injure spinal cord. These shortcomings should be noticed before utilizing ultrasonic osteotome.
We have shown the feasibility of an ultrasonic osteotome for thoracic beak-type OPLL. We hope that surgical difficulties might be overcome by utilizing this technique. However, a study with a longer follow-up and a larger number of patients is required to show effectiveness of the present surgical technique.
Acknowledgements
CKC : This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2010-0028631). CHK : This work was supported by Grant No. 0420133010 (2013-1098) from the Seoul National University Hospital Research Fund. This study was approved by the institutional review board at the Seoul National University Hospital (H-1412-008-618).
References
1. Bolesta MJ, Caron T, Chinthakunta SR, Vazifeh PN, Khalil S. Pedicle screw instrumentation of thoracolumbar burst fractures : biomechanical evaluation of screw configuration with pedicle screws at the level of the fracture. Int J Spine Surg. 2012; 6:200–205. PMID: 25694892.

2. Chaichana KL, Jallo GI, Dorafshar AH, Ahn ES. Novel use of an ultrasonic bone-cutting device for endoscopic-assisted craniosynostosis surgery. Childs Nerv Syst. 2013; [Epub ahead of print].

3. Epstein NE. Medicolegal corner : when minimally invasive thoracic surgery leads to paraplegia. Surg Neurol Int. 2014; 5(Suppl 3):S55–S58. PMID: 24843811.
4. Fujimura Y, Nishi Y, Nakamura M, Toyama Y, Suzuki N. Long-term follow-up study of anterior decompression and fusion for thoracic myelopathy resulting from ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 1997; 22:305–311. PMID: 9051893.

5. Hanai K, Ogikubo O, Miyashita T. Anterior decompression for myelopathy resulting from thoracic ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2002; 27:1070–1076. PMID: 12004174.

6. Hosono N, Sakaura H, Mukai Y, Ishii T, Yoshikawa H. En bloc laminoplasty without dissection of paraspinal muscles. J Neurosurg Spine. 2005; 3:29–33. PMID: 16122019.

7. Hu X, Ohnmeiss DD, Lieberman IH. Use of an ultrasonic osteotome device in spine surgery : experience from the first 128 patients. Eur Spine J. 2013; 22:2845–2849. PMID: 23584231.

8. Kawahara N, Tomita K, Murakami H, Hato T, Demura S, Sekino Y, et al. Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2008; 33:39–46. PMID: 18165747.

9. Kimura H, Fujibayashi S, Takemoto M, Otsuki B, Matsuda S. Spontaneous reduction in ossification of the posterior longitudinal ligament of the thoracic spine after posterior spinal fusion without decompression : a case report. Spine (Phila Pa 1976). 2014; 39:E417–E419. PMID: 24384668.
10. Lee SE, Jahng TA, Chung CK, Kim HJ. Circumferential spinal cord decompression through a posterior midline approach with lateral auxiliary ports for lower thoracic compressive myelopathy. Neurosurgery. 2012; 70(2 Suppl Operative):221–229. PMID: 21937940.

11. Liu X, Zhu B, Liu X, Liu Z, Dang G. Circumferential decompression via the posterior approach for the surgical treatment of multilevel thoracic ossification of the posterior longitudinal ligaments : a single institution comparative study. Chin Med J (Engl). 2014; 127:3371–3377. PMID: 25269897.
12. Ma X, An HS, Zhang Y, Brown NM, Chen Z, Zhang G, et al. A radical procedure of circumferential spinal cord decompression through a modified posterior approach for thoracic myelopathy caused by severely impinging anterior ossification. Spine J. 2014; 14:651–658. PMID: 24161362.

13. Matsumoto M, Chiba K, Toyama Y, Takeshita K, Seichi A, Nakamura K, et al. Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine : a multi-institutional retrospective study. Spine (Phila Pa 1976). 2008; 33:1034–1041. PMID: 18427326.

14. Matsumoto M, Toyama Y, Chikuda H, Takeshita K, Kato T, Shindo S, et al. Outcomes of fusion surgery for ossification of the posterior longitudinal ligament of the thoracic spine : a multicenter retrospective survey : clinical article. J Neurosurg Spine. 2011; 15:380–385. PMID: 21740130.

15. Matsuyama Y, Sakai Y, Katayama Y, Imagama S, Ito Z, Wakao N, et al. Indirect posterior decompression with corrective fusion for ossification of the posterior longitudinal ligament of the thoracic spine : is it possible to predict the surgical results? Eur Spine J. 2009; 18:943–948. PMID: 19347374.

16. Matsuyama Y, Yoshihara H, Tsuji T, Sakai Y, Yukawa Y, Nakamura H, et al. Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine : implication of the type of ossification and surgical options. J Spinal Disord Tech. 2005; 18:492–497. discussion 498PMID: 16306836.

17. McClendon J Jr, Sugrue PA, Ganju A, Koski TR, Liu JC. Management of ossification of the posterior longitudinal ligament of the thoracic spine. Neurosurg Focus. 2011; 30:E16. PMID: 21361754.

18. Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech. 2008; 21:116–119. PMID: 18391716.

19. Miyashita T, Ataka H, Tanno T. Spontaneous reduction of a floated ossification of the ligamentum flavum after posterior thoracic decompression (floating method); report of a case (abridged translation of a primary publication). Spine J. 2013; 13:e7–e9. PMID: 23490728.

20. Nickele C, Hanna A, Baskaya MK. Osteotomy for laminoplasty without soft tissue penetration, performed using a harmonic bone scalpel : instrumentation and technique. J Neurol Surg A Cent Eur Neurosurg. 2013; 74:183–186. PMID: 23504672.

21. Onishi E, Sano H, Matsushita M. Surgical treatment for thoracic myelopathy due to simultaneous ossification of the posterior longitudinal ligament and ligamentum flavum at the same level. J Spinal Disord Tech. 2013; [Epub ahead of print].

22. Park SB, Jahng TA, Kim CH, Chung CK. Thoracic and lumbar laminoplasty using a translaminar screw : morphometric study and technique. J Neurosurg Spine. 2009; 10:603–609. PMID: 19558295.

23. Sanborn MR, Balzer J, Gerszten PC, Karausky P, Cheng BC, Welch WC. Safety and efficacy of a novel ultrasonic osteotome device in an ovine model. J Clin Neurosci. 2011; 18:1528–1533. PMID: 21917459.

24. Schaeren S, Jaquiéry C, Heberer M, Tolnay M, Vercellotti T, Martin I. Assessment of nerve damage using a novel ultrasonic device for bone cutting. J Oral Maxillofac Surg. 2008; 66:593–596. PMID: 18280402.

25. Sugita S, Chikuda H, Takeshita K, Seichi A, Tanaka S. Progression of ossification of the posterior longitudinal ligament of the thoracic spine following posterior decompression and stabilization. J Neurosurg Spine. 2014; 21:773–777. PMID: 25127433.

26. Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Minami A. Clinical results and complications of circumferential spinal cord decompression through a single posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine (Phila Pa 1976). 2008; 33:1199–1208. PMID: 18469693.

27. Tomita K, Kawahara N, Baba H, Kikuchi Y, Nishimura H. Circumspinal decompression for thoracic myelopathy due to combined ossification of the posterior longitudinal ligament and ligamentum flavum. Spine (Phila Pa 1976). 1990; 15:1114–1120. PMID: 2125147.

28. Yamazaki M, Mochizuki M, Ikeda Y, Sodeyama T, Okawa A, Koda M, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament : operative indication of posterior decompression with instrumented fusion. Spine (Phila Pa 1976). 2006; 31:1452–1460. PMID: 16741454.

29. Yang SH, Kim CH, Chung CK, Park SB, Sohn S, Lee S. Bone fusion rate in the thoracic and lumbar spine after laminoplasty with laminar screws. Spine (Phila Pa 1976). 2014; 39:E1325–E1330. PMID: 25188592.

30. Zhang HQ, Chen LQ, Liu SH, Zhao D, Guo CF. Posterior decompression with kyphosis correction for thoracic myelopathy due to ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament at the same level. J Neurosurg Spine. 2010; 13:116–122. PMID: 20594026.

SUPPLEMENTARY MATERIALS
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3340/jkns.2015.58.6.571
Fig. 1
Ultrasonic osteotome. Various kinds of tips could be attached to the hand piece according to the purpose of surgery (A). The blade (B, left) was used for laminotomy and shaving tips (B, middle and right) were preferentially used in direct removal of ossified mass.

Fig. 2
A : The lamina was gradually cut with ultrasonic osteotome by slight pushing down its blade against lamina with a vertical motion (right blade). When inner cortical bone was cut, loss of resistance was felt (left blade) and the ostetome was not inserted beyond the depth. Then the ostetome was moved cranially and caudally along the lamina for laminotomy. B : After laminotomy, facetectomy and pediclectomy, ossification of posterior longitudinal ligament (OPLL) mass was exposed under the dura (white broken line). The ultrasonic osteotome was inserted at the base of OPLL mass and moved gradually along the OPLL mass (white curved arrow). Note that nerve root was retracted with the shaft of ultrasonic osteotome. Because the energy was not transmitted from the shaft, the osteotome could be inserted at the base of OPLL mass while retracting nerve root.
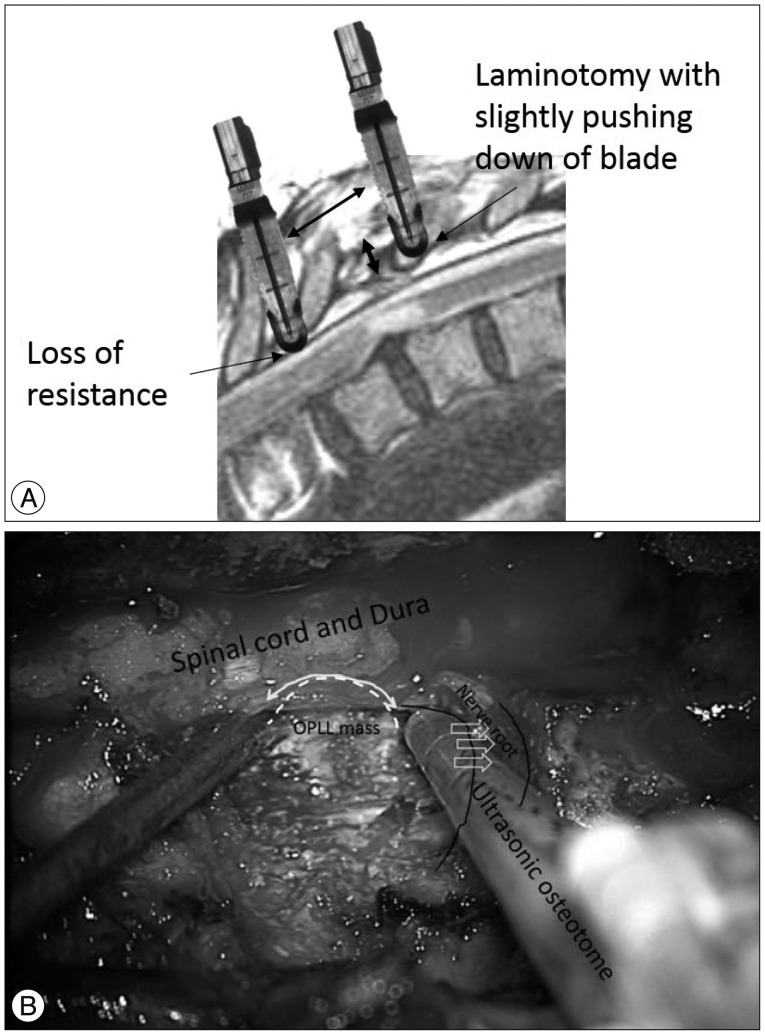
Fig. 3
Case 3. Sagittal magnetic resonance (MR) T2-weighted imaging (A), sagittally reconstructed (B), axial (C) and 3-D (D) computed tomography (CT) scan showed beak-type ossified posterior longitudinal ligament (OPLL). The level of the axial scan was marked in sagittal CT scan with a black line. Postoperative sagittally reconstructed CT scan (E) showed complete removal of OPLL mass (F, white line in E), but a small fragment was left at T7 (G, arrow, black line in E). A 3-D reconstructed CT scan showed instrumentation, removal of OPLL (H, arrow) and laminoplasty with miniplate (I).

Fig. 4
Case 4. Sagittal magnetic resonance (MR) T2-weighted imaging (A), sagittally reconstructed (B), and axial (C) computed tomography (CT) scan showed beak-type ossified posterior longitudinal ligament (OPLL). Postoperative sagittal MR imaging (D) and sagittal (E) and axial CT scans (F) showed complete removal of T-OPLL mass. The laminoplasty was performed with translaminar screws (G).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download