Abstract
Acute subdural hematoma (SDH) of arterial origin is rare, especially SDH associated with an arteriovenous malformation (AVM) is extremely rare. The authors report a case of acute spontaneous SDH due to rupture of a tiny cortical AVM. A 51-year-old male presented with sudden onset headache and mentality deterioration without a history of trauma. Brain CT revealed a large volume acute SDH compressing the right cerebral hemisphere with subfalcine and tentorial herniation. Emergency decompressive craniectomy was performed to remove the hematoma and during surgery a small (5 mm sized) conglomerated aciniform mass with two surrounding enlarged vessels was identified on the parietal cortex. After warm saline irrigation of the mass, active bleeding developed from a one of the vessel. The bleeding was stopped by coagulation and the vessels were removed. Histopathological examination confirmed the lesion as an AVM. We concluded that a small cortical AVM existed at this area, and that the cortical AVM had caused the acute SDH. Follow up conventional angiography confirmed the absence of remnant AVM or any other vascular abnormality. This report demonstrates rupture of a cortical AVM is worth considering when a patient presents with non-traumatic SDH without intracerebral hemorrhage or subarachnoid hemorrhage.
Go to : 
Acute spontaneous subdural hematoma (SDH) is very rare, and usually caused by tearing of the bridging veins in the subdural space369). Furthermore, bleeding from cerebral surface cortical arteries is extremely rare. The pathophysiology of spontaneous SDH includes rupture of the cortical artery or of the pial arteriovenous fistulae, an arteriovenous malformation (AVM), or intracranial aneurysm1269). Here, we reported a very rare case of acute spontaneous SDH due to rupture of a tiny cortical AVM that was pathologically shown to be an AVM.
Go to : 
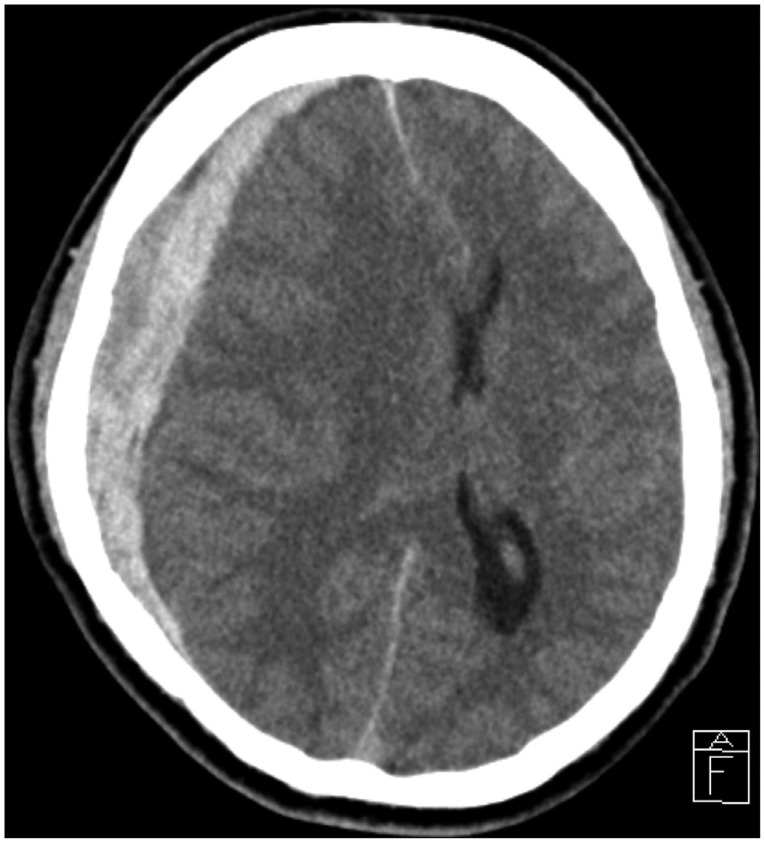
A 51-year-old man presented with a sudden onset of headache and gradually diminishing mentality. On neurological examination of the patient at admission time was semicomatous. There was no recent medical history of head trauma or notable disease. Neurological examination showed Glasgow Coma Scale (GCS) 5, fixed light reflex, and dilatation of both pupils. A computerized tomography (CT) of the brain depicted an acute SDH with severe midline shift in the right cerebral hemisphere (Fig. 1). However, we did not performed another imaging study, such as a CT angiography or conventional cerebral angiography. Laboratory findings, including blood analysis, blood cell counts and coagulation cascades, were normal.
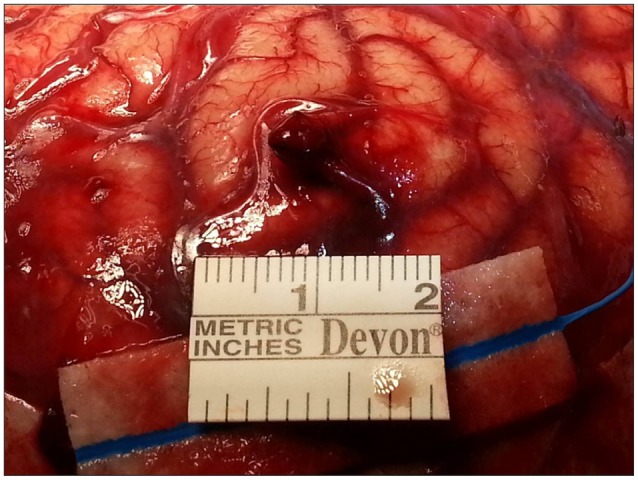
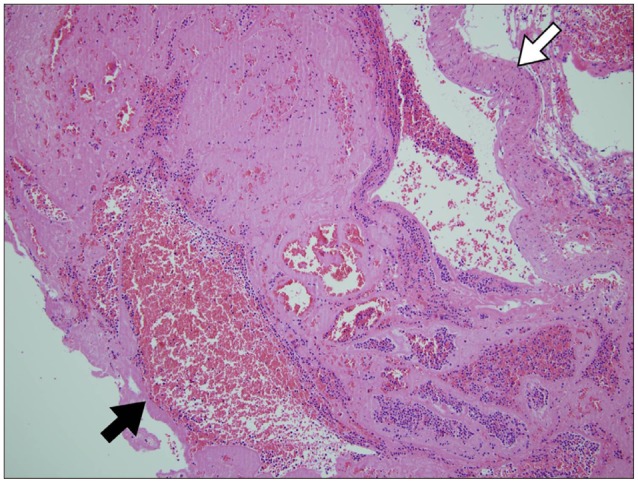
Emergent decompressive craniectomy was conducted to remove the SDH. The bone flap and the dura mater revealed no evidence of head trauma, such as, bone fracture or vascular abnormality. The subdural blood clot was removed and a tiny (5 mm sized) aciniform mass with two connected engorged vessels was identified on parietal lobe (Fig. 2). In addition, warm saline irrigation of the mass resulted in active pulsatile bleeding. Accordingly, both vessels were coagulated and the mass was totally removed. Postoperative histopathological examination confirmed the lesion as a malformed vessel with thrombus (Fig. 3). Subsequent cerebral angiography demonstrated the absence of remnant AVM or another vascular abnormality.
After operation, mild neurologic deterioration with hemiparesis persisted, but his symptoms were considerably improved with a Glasgow Outcome Scale 3.
Go to : 
Damage of bridging veins in the subdural space is the most common cause of acute and chronic SDH1). However, in the absence of trauma injury to the brain, spontaneous bleeding may occur due to ruptures of small cortical arteries or intracranial aneurysms, hypertensive cerebral hemorrhage, tumors, and hematologic disorders1269). Non-traumatic spontaneous SDH accounts for 25% of all SDH cases410).
Some authors have proposed mechanisms for the development of acute spontaneous SDH. Drake3) concluded that the acute or subacute SDH could result from a small rent in a surface cortical artery torn from a dural attachment by the movement of brain. Kondziolka et al.6) suggested an aneurysm adherent to the arachnoid membrane might bleed directly into the subdural space or hemorrhage under high pressure and might lead to pia-arachnoid rupture and extravasation of blood into the subdural space. McDermott et al.9) described some anatomical situations that might predispose spontaneous rupture of a cortical artery, that is, rupture of a cortical artery at the bleeding origin of a fragile arterial twig or rupture of a small artery crossing the subdural space and connecting a cortical artery to the dura mater (a bridging artery).
AVMs are vascular abnormalities composed of fistulous vessels between arteries and veins without a normal capillary bed. McCormick et al.8) classified vascular malformations into five subgroups, namely, cavernous malformations, telangiectasia, varix, venous malformation, and AVMs. And he found that AVMs were more likely to bleed.
The annual hemorrhagic risk posed by an AVM is 2-3%, and the annual risk of fatality is 1%5). Furthermore, AVMs are associated with a definite risk of neurologic deficit with or without a bleeding events. Other presenting symptoms include hydrocephalus, raised intracranial pressure, papilledema, ataxia, motor weakness, and impaired consciousness27).
AVMs are most commonly located in the supratentorially, but are also encountered in cerebellar hemispheres and vermis7). Almost cases with cranial AVMs, more than half of patients present with hemorrhage2). Intraparenchymal hemorrhage is the most common, followed by intraventricular hemorrhage (IVH) and subarachnoid hemorrhage (SAH). However, a presentation of SDH due to a ruptured cerebral AVM is extremely rare. In fact, only one case of acute posterior fossa SDH following ruptured cerebellar AVM rupture has been previously reported2). These patients are suffering from the signs and symptoms of intraparenchymal hemorrhage, IVH and SAH , because of almost AVMs are located in the subcortical area and triangular shape with the base toward the cerebral cortex and the apex toward the ventricular system. However, in the present case, we encountered subdural bleeding through a hole in the cortical AVM. We thought the adhesion of the AVM vessel to the arachnoid membrane and strain of arachnoid membrane could be led formation of SDH rather than SAH or intraparenchymal hemorrhage.
In the case of our patient, the pulsatile bleeding focus was at a hole on the wall of arterialized vein with an extraordinary morphology. However, it should be noted a focus of bleeding in the operation field is not guaranteed in cases of acute spontaneous SDH, and thus, a close inspection of the cerebral cortex should be conducted intraoperatively in cases of acute spontaneous SDH without a traumatic history.
Medical history and physical examination provide important diagnostic clue, but a confirmatory imaging study by CT, MRI, and angiography is required. In our opinion, brain CT is the most valuable modality for determining treatment strategy in urgent situations, which often means, there is no time for other imaging studies. However, when the situation permits, we recommend CT angiography in cases of acute spontaneous SDH without a definite trauma history. An extravasation of contrast media from a cortical vessel is helpful for localizing the bleeding focus and detection of cortical AVM.
Go to : 
We report an extremely rare case of acute spontaneous SDH due to rupture of a tiny cortical AVM. The CT angiography should be used to locate the bleeding focus on patients with spontaneous acute SDH without a definite traumatic history. Furthermore, a careful inspection of the brain cortex below the hematoma should be conducted during the surgery.
Go to : 
References
1. Byun HS, Patel PP. Spontaneous subdural hematoma of arterial origin : report of two cases. Neurosurgery. 1979; 5:611–613. PMID: 534070.
2. Datta NN, Chan KY, Kwok JC, Poon CY. Posterior fossa subdural hematoma due to ruptured arteriovenous malformation. Case report. Neurosurg Focus. 2000; 8:ecp1. PMID: 16859278.
3. Drake CG. Subdural haematoma from arterial rupture. J Neurosurg. 1961; 18:597–601. PMID: 13724255.

4. Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma : surgical treatment and outcome in 104 patients. Surg Neurol. 1997; 48:220–225. PMID: 9290707.

5. Guazzo EP, Xuereb JH. Spontaneous thrombosis of an arteriovenous malformation. J Neurol Neurosurg Psychiatry. 1994; 57:1410–1412. PMID: 7964822.

6. Kondziolka D, Bernstein M, ter Brugge K, Schutz H. Acute subdural hematoma from ruptured posterior communicating artery aneurysm. Neurosurgery. 1988; 22(1 Pt 1):151–154. PMID: 3344078.

7. Matsumura H, Makita Y, Someda K, Kondo A. Arteriovenous malformations in the posterior fossa. J Neurosurg. 1977; 47:50–56. PMID: 301180.

8. McCormick WF, Hardman JM, Boulter TR. Vascular malformations ("angiomas") of the brain, with special reference to those occurring in the posterior fossa. J Neurosurg. 1968; 28:241–251. PMID: 5643915.

9. McDermott M, Fleming JF, Vanderlinden RG, Tucker WS. Spontaneous arterial subdural hematoma. Neurosurgery. 1984; 14:13–18. PMID: 6694787.

10. Sambasivan M. An overview of chronic subdural hematoma : experience with 2300 cases. Surg Neurol. 1997; 47:418–422. PMID: 9131021.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download