Abstract
Background
Cerebral ischemia is as a result of insufficient cerebral blood flow for cerebral metabolic functions. Resveratrol is a natural phytoalexin that can be extracted from grape's skin and had potent role in treating the cerebral ischemia. Apoptosis, a genetically programmed cellular event which occurs after ischemia and leads to biochemical and morphological changes in cells. There are some useful markers for apoptosis like Bcl-2, bax, and p53. The last reports, researchers verify the apoptosis with early markers like Annexin V.
Methods
We preferred in this experimental study a model of global cerebral infarction which was induced by bilateral common carotid artery occlusion method. Rats were randomly divided into 4 groups : sham, ischemia-reperfusion (I/R), I/R plus 20 mg/kg resveratrol and I/R plus 40 mg/kg resveratrol. Statistical analysis was performed using Sigmastat 3.5 ve IBM SPSS Statistics 20. We considered a result significant when p<0.001.
Results
After administration of resveratrol, Bcl-2 and Annexin levels were significantly increased (p<0.001). Depending on the dose of resveratrol, Bcl2 levels increased, p53 levels decreased but Annexin V did not effected. P53 levels were significantly increased in ishemia group, so apoptosis is higher compared to other groups.
Cerebral ischemia is as a result of insufficient cerebral blood flow for cerebral metabolic functions. Stroke is the second leading cause of long-term disability and death worldwide67). Oxidative stress and inflammation have an important role in cerebral infarction which mediated by ishemia and reperfusion1826). Reperfusion injury stimulates many pathological mechanisms such as leukocyte infiltration, oxidative stress, inflammation, destruction of blood-brain barrier, platelet activation, nitric oxide release, and apoptosis293134). Consequently, a potent anti-inflammatory and antioxidant mediators may be beneficial in the treatment of cerebral ischemia and reperfusion injury. Mitochondria produces ATP, is essential for cell function, and plays an important role in response to oxidative stres8). Energy failure is due to insufficient ATP for a cell to maintain its cellular functions or defenses against oxidative stresses32). Pathological events like ischemia cause necrosis but can also induce apoptosis20). Severe oxidative stress causes necrosis, and milder stress causes apoptosis. Increased oxidative stress cause reduced ATP production in mitochondria, and this releases cytochrome C an AIF, which leads to apoptosis.
Recent researches prove that activation of the cysteine protease caspases is critical for apoptosis in ischemia-induced brain injury21). During apoptosis caspases are activated in the intracellular region and leads to degradation of cellular constituents; finally leading to cell death. Caspase-3 is believed to be the main executioner protease of apoptotic caspase36).
Resveratrol is a natural phytoalexin that can be extracted from grape's skin. Resveratrol was reported to have antioxidant, antiinflammatory, antidiabetic, and antiaging, control of cell cycle, and apoptosis112849). Earlier reports suggested that antioxidants and anti-inflammatory agents had potent role in treating the cerebral ischemia-reperfusion injury549). Antiphospholipid antibodies (aPLs) are associated with some neurologic disorders. Annexins are a family of calcium-dependent phospholipid-binding proteins. The potent anticoagulant activity of annexin V is derived from its inhibitory effect on prothrombin activation3242). Antiannexin V antibody (aAV) has an important role in trombotic events in patients with systemic lupus erythematosus and antiphospholipid syndrome12) aAV plays a role in cerebral thrombotic events by inhibiting the anticoagulant activity of annexin V16).
p53 is an important modulator of cell death and survival, and its inhibition could be a therapeutic approach to several neuropathologies9). Bcl-2, a gene located at chromosome 18q21, blocks apoptosis without affecting cellular proliferation. Increases in the expression of bcl-2 stop apoptosis and inhibits neuron death. p53 induced apoptosis activate Bax and cause neuronal apoptosis41). Bcl-2 is a distinguished anti-apoptotic protein that binds to Bax to inhibit apoptosis24). p53 induced downregulation of Bcl-2 and upregulation of Bax promote cytochrome C release from the mitochondria and caspase-3 activation causes cell death1435).
In this study we have aimed to show the effects of resveratrol with the comparison results of annexin V, p53, Bcl-2 levels after ischemia in rats.
All experimental procedures and animal care were in accordance with the guidelines of Eskisehir Osmangazi University, Faculty of Medicine. The experimental protocols used in this study were also approved by the Animal Experimental Ethic Committee of Eskişehir Osmangazi University (Date and decision number : 25.07.2013, 351).
Rat Annexin V ELISA kit (sc 4252-AK, Santa Cruz Biotechnology, TX, USA), rat p53 ELISA kit (Cloud-Clone Corp., Houston, TX, USA), rat Bcl-2 ELISA kit (Cloud-Clone Corp., Houston, TX, USA) and resveratrol (Sigma-Aldrich, St, Louis, MO, USA) were used for measuring the serum levels of these apoptotic markers.
All efforts were made to minimize animal suffering and to reduce the number of animals used. In the present study, 50 Sprague-Dawley female rats (250-300 g weight) were used. The rats were monitored at a suitable temperature (21±2℃) and moist environment (60±5% humidity). Rats was observed in this environment for one week before starting the experiment to adapt to the environment.
The animals included in the study were divided into five groups (Table 1). Group I, Sham, no ischemia (n=10), Group II : bilateral carotid artery (BCA) occlusion for 30 min (n=10), no resveratrol; Group III, BCA occlusion for 30 min; followed by 20 mg/kg resveratrol i.p. (n=10) followed by reperfusion for 24 h (n=10); Group IV, BCA occlusion for 30 min, followed by 40 mg/kg resveratrol i.p. (n=10); Group V, BCA occlusion for 30 min, followed by DMSO (solvent).
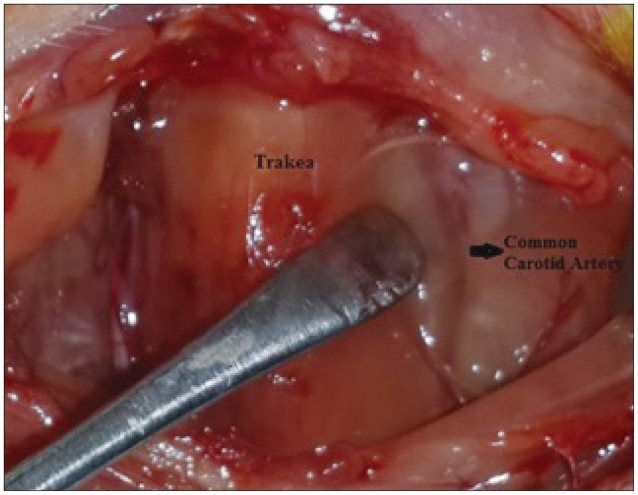
In this experimental study, we preferred a model of global cerebral infarction which was induced by bilateral common carotid artery occlusion method (Fig. 1)10).
Rats were anesthetized with thiopental sodium (30 mg/kg). Cervical vertebrae and the common carotid arteries were then exposed and carefully separated from the vagus nerve. These arteries were occluded for 30 min followed by reperfusion for 4 h. The rectal temperature was maintained at 37±0.5 ℃ with a feedback-controlled heating pad. animals which did not lose the righting reflex or which convulsed during the ischemic episode were excluded.
Statistical analysis was performed using Sigmastat 3.5 and IBM SPSS Statistics 21. Continuous variables are expressed as mean±standard deviation. Relationships between Bcl-2 and p53 levels were determined using analysis of variance (ANOVA). The variables did not show normal distribution in comparison between Annexin V levels, we used Kruskal-Wallis one-way analysis of variance. As multiple comparison test annexin V levels were measured by Tukey's test. We considered a significant result when p<0.001.
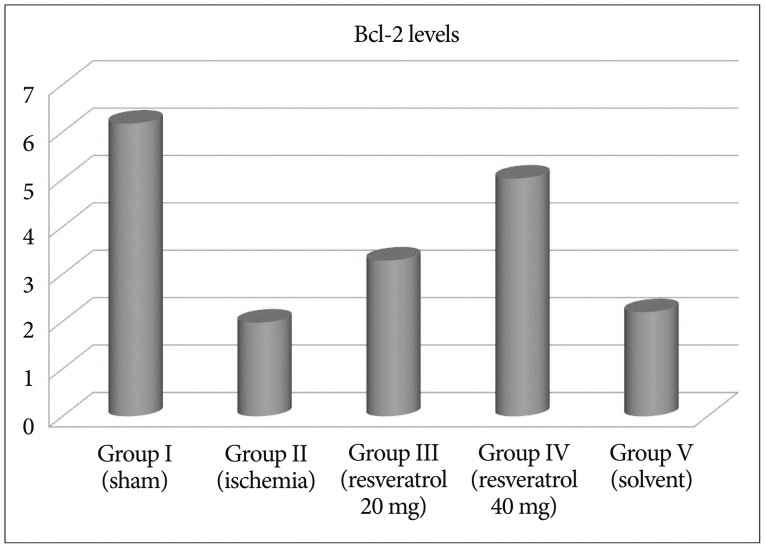
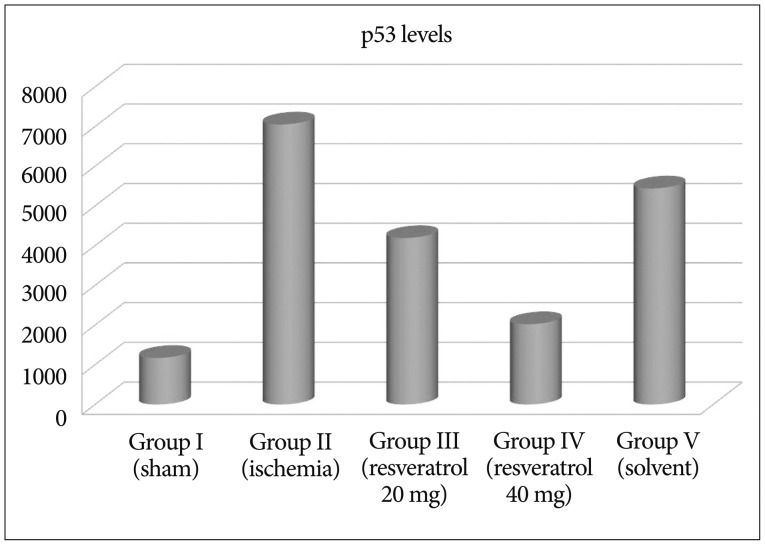
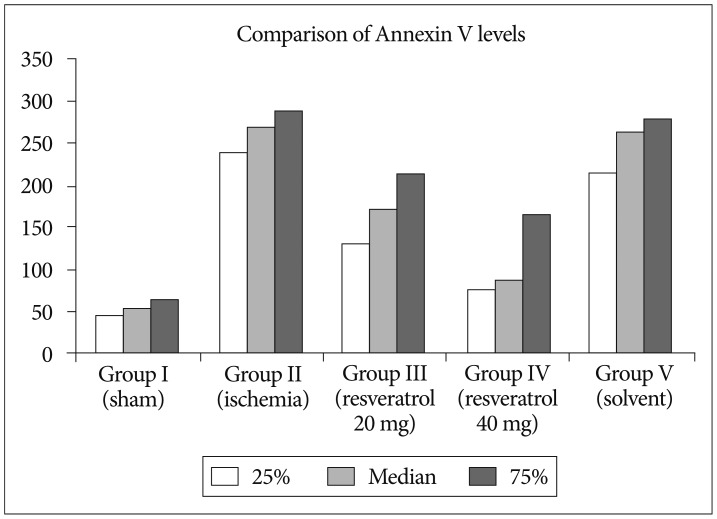
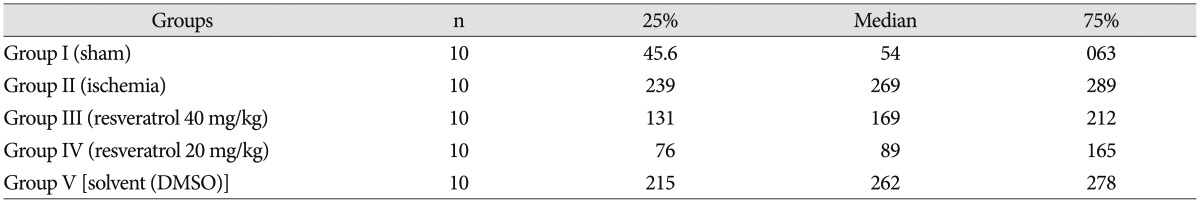
After resveratrol administration, Bcl-2 levels were significantly increased when compared to the ishemia group (p<0.001). Depending on the dose of resveratrol, we have observed that Bcl-2 levels increase. We have found that the solvent did not change Bcl-2 levels (Fig. 2). p53 levels were significantly increased in ishemia group, so apoptosis is higher compared to other groups. Depending on the dose of resveratrol, we observed that p53 levels decrease so the apoptosis (Fig. 3). Our data revealed that Annexin V levels were significantly increased in ischemia group compared to other groups. However, depending on the dose of resveratrol dsaAnnexin V levels did not change (Fig. 4, Table 2).
The annual incidence of stroke around the world is 15 million, with a mortality of 5 million and permanent sequelae, disability and dependency on others of 5 million patients23). It is estimated that the stroke rate will rise significantly in the future45). During the stroke, several mechanisms are responsible for the neuronal injury including increased excitotoxicity, calcium overload, formation of free radicals and inhibition of protein synthesis3346). During ischemia/reperfusion, resveratrol modulate the neurotransmitters and neuromodulator protects the brain from ischemia17). Li et al.18) showed that treatment with resveratrol improve neurological function and decrease infarct volume as compared to ischemia rats. The neuroprotective mechanism of resveratrol are not clarified and further investigation must be conducted18). Apoptosis, inflammatory response, and free radical formation are responsible for the neuronal cell death. Apoptosis, a programmed cell death, activated by the cell ischemia1). A family of proteases known as caspases plays an important role in apoptosis. There are two ways of caspase activation : cell death receptor mediated and mitochondrial. The Bcl-2 family includes both antiapoptotic proteins (Bcl-2, Bcl-xL, A1/Bfl1, Boo/Diva, Bcl-w, Mcl-1, Nrf3) and proapoptotic proteins (Bax, Bak, Bok/Mtd)17). Cytochrome C induces cell death by activation of caspase 9 and 3 in the mitochondrial pathway of apoptosis4). The mitochondrial release of cytochrome C in brain ischemia is caused by proapototic Bax gene, but it's not the only way of apoptosis. Apoptosis can be activated in the absence of caspase activity. Apoptosis-inducing factor (AIF), which is found in the mitochondrial intermembranous space, releases as a result of absence of ATP, causing apoptosis3).
Antioxidant and anti-inflammatory agents can limit the reperfusion injury during the ishemia13153348). Some research showed that resveratrol has a neuroprotective effects in rats293947). There is no standard treatment for the reperfusion injury. Oxidative stress and inflammation have an important role in cerebral ischemia and reperfusion injury1530). Resveratrol has a neuroprotective effect in cerebral ischemic models3944). Resveratrol can prevent a wide variety of conditions including cancer, cardiovascular, diabetes and neurological disorders 22543). Resveratrol exerts multiple biological effects, including anti-inflammatory, antiproliferative, antiplatelet and potent antioxidant effects411).
The present study indicates that resveratrol presents a neuroprotective effect on cerebral ischemic rats by measuring Annexin 5, p53, and bcl-2. Li et al.19) indicated that resveratrol attenuates ischemic neuronal apoptosis by up regulating bcl-2. In our study after resveratrol administration, Bcl-2 levels were significantly increased when compared to the ishemia group. Shin et al.38) showed that in the ischemic brain, resveratrol modulate the proteins and protects the brain. Annexin 5 has been extensively used as an indicator of early apoptosis due to its inherent property of selectively interacting with phosphatidyl serine. The physiologic functions of Annexin 5 include suppression of blood coagulation and platelet aggregation by occlusion of exposed PS from access by coagulation factors37). In our study we found that Annexin V levels were significantly increased in ischemia group compared to other groups. However, depending on the dose of resveratrol Annexin V levels did not change. Resveratrol affects the activation of a variety of intracellular signal pathways. Molchadsky et al.27) showed that resveratrol induces p53 activation in vascular smooth muscle cells (VSMCs ). Unlike Molchadsky et al.27), Zheng et al.50) showed that p53 decreased myocardin expression; hence inhibited differentiation from mesenchymal cells to smooth muscle cells. We observed that p53 levels decreases after the administration of resveratrol. Orsu et al.30) indicated that resveratrol showed significant cerebroprotective action mediated by anti-oxidant and anti-inflammatory mechanisms. Lu et al.22) suggest that resveratrol prepared by RES loaded nanoparticles shows enhanced neuron protection against oxidative stress in cultured neurons exposed to H2O2 both in a short time and in longer time treatment over bax and bcl 2 genes.
In conclusion, we suggest that resveratrol is a natural drug with minimal side effects and it might be effective medical theraphy for the treatment of acute ischemic stroke.
References
1. Aboutaleb N, Shamsaei N, Khaksari M, Erfani S, Rajabi H, Nikbakht F. Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. J Physiol Sci. 2015; 65:435–443. PMID: 26012958.

2. Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer : preclinical and clinical studies. Anticancer Res. 2004; 24(5A):2783–2840. PMID: 15517885.
3. Choi SK, Lee GJ, Choi S, Kim YJ, Park HK, Park BJ. Neuroprotective effects by nimodipine treatment in the experimental global ischemic rat model : real time estimation of glutamate. J Korean Neurosurg Soc. 2011; 49:1–7. PMID: 21494355.

4. de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent : mechanisms and clinical implications. Biochem Soc Trans. 2007; 35(Pt 5):1156–1160. PMID: 17956300.
5. del Zoppo GJ. Microvascular responses to cerebral ischemia/inflammation. Ann N Y Acad Sci. 1997; 823:132–147. PMID: 9292040.

8. Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012; 322:254–262. PMID: 22669122.

9. Green DR, Chipuk JE. p53 and metabolism : inside the TIGAR. Cell. 2006; 126:30–32. PMID: 16839873.
10. Iwasaki Y, Ito S, Suzuki M, Nagahori T, Yamamoto T, Konno H. Forebrain ischemia induced by temporary bilateral common carotid occlusion in normotensive rats. J Neurol Sci. 1989; 90:155–165. PMID: 2723680.

11. Juhasz B, Varga B, Gesztelyi R, Kemeny-Beke A, Zsuga J, Tosaki A. Resveratrol : a multifunctional cytoprotective molecule. Curr Pharm Biotechnol. 2010; 11:810–818. PMID: 20874691.
12. Kaburaki J, Kuwana M, Yamamoto M, Kawai S, Ikeda Y. Clinical significance of anti-annexin V antibodies in patients with systemic lupus erythematosus. Am J Hematol. 1997; 54:209–213. PMID: 9067499.

13. Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004; 76:519–527. PMID: 15114624.

14. Lahiry L, Saha B, Chakraborty J, Bhattacharyya S, Chattopadhyay S, Banerjee S, et al. Contribution of p53-mediated bax transactivation in theaflavin-induced mammary epithelial carcinoma cell apoptosis. Apoptosis. 2008; 13:771–781. PMID: 18454316.

15. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke : therapeutic approaches. J Transl Med. 2009; 7:97. PMID: 19919699.
16. Lee KO, Kim WJ, Na SJ, Heo JH, Lee KY. Clinical significance of anti-annexin V antibody in acute cerebral ischemia. J Neurol Sci. 2011; 305:53–56. PMID: 21450308.

17. Li C, Yan Z, Yang J, Chen H, Li H, Jiang Y, et al. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochem Int. 2010; 56:495–500. PMID: 20026214.

18. Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011; 60:252–258. PMID: 20868700.

19. Li Z, Pang L, Fang F, Zhang G, Zhang J, Xie M, et al. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 2012; 1450:116–124. PMID: 22410291.

20. Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol. 1998; 274(2 Pt 2):F315–F327. PMID: 9486226.
21. Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27:267–282. PMID: 12657366.

22. Lu X, Xu H, Sun B, Zhu Z, Zheng D, Li X. Enhanced neuroprotective effects of resveratrol delivered by nanoparticles on hydrogen peroxide-induced oxidative stress in rat cortical cell culture. Mol Pharm. 2013; 10:2045–2053. PMID: 23534345.

23. Mackay J, Mensah GA, Mendis S, Greenlund K. The atlas of heart disease and stroke. Geneva: World Health Organization;2004.
24. Mahajan NP, Linder K, Berry G, Gordon GW, Heim R, Herman B. Bcl-2 and bax interactions in mitochondria probed with green fluorescent protein and fluorescence resonance energy transfer. Nat Biotechnol. 1998; 16:547–552. PMID: 9624685.

25. Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008; 3:331–339. PMID: 18686754.
26. Mohagheghi F, Khalaj L, Ahmadiani A, Rahmani B. Gemfibrozil pretreatment affecting antioxidant defense system and inflammatory, but not Nrf-2 signaling pathways resulted in female neuroprotection and male neurotoxicity in the rat models of global cerebral ischemia-reperfusion. Neurotox Res. 2013; 23:225–237. PMID: 22773136.

27. Molchadsky A, Shats I, Goldfinger N, Pevsner-Fischer M, Olson M, Rinon A, et al. p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS One. 2008; 3:e3707. PMID: 19002260.

28. Mukherjee PK, Ahamed KF, Kumar V, Mukherjee K, Houghton PJ. Protective effect of biflavones from araucaria bidwillii hook in rat cerebral ischemia/reperfusion induced oxidative stress. Behav Brain Res. 2007; 178:221–228. PMID: 17250903.

29. Nassar NN, Abdelsalam RM, Abdel-Rahman AA, Abdallah DM. Possible involvement of oxidative stress and inflammatory mediators in the protective effects of the early preconditioning window against transient global ischemia in rats. Neurochem Res. 2012; 37:614–621. PMID: 22113727.

30. Orsu P, Murthy BV, Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J Neural Transm (Vienna). 2013; 120:1217–1223. PMID: 23371441.

31. Park HS, Han KH, Shin JA, Park JH, Song KY, Kim DH. The neuroprotective effects of carnosine in early stage of focal ischemia rodent model. J Korean Neurosurg Soc. 2014; 55:125–130. PMID: 24851146.

32. Pathak D, Berthet A, Nakamura K. Energy failure : does it contribute to neurodegeneration? Ann Neurol. 2013; 74:506–516. PMID: 24038413.
33. Phillis JW, O'Regan MH. Characterization of modes of release of amino acids in the ischemic/reperfused rat cerebral cortex. Neurochem Int. 2003; 43:461–467. PMID: 12742092.

34. Prabhakar O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2013; 386:705–710. PMID: 23612842.

35. Qin ZH, Chen RW, Wang Y, Nakai M, Chuang DM, Chase TN. Nuclear factor kappaB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci. 1999; 19:4023–4033. PMID: 10234031.

36. Rami A. Ischemic neuronal death in the rat hippocampus : the calpain-calpastatin-caspase hypothesis. Neurobiol Dis. 2003; 13:75–88. PMID: 12828932.

37. Raynal P, Pollard HB. Annexins : the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994; 1197:63–93. PMID: 8155692.

38. Shin JA, Lee KE, Kim HS, Park EM. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochem Res. 2012; 37:2686–2696. PMID: 22878646.

39. Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002; 71:655–665. PMID: 12072154.

40. Slemmer JE, Shacka JJ, Sweeney MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008; 15:404–414. PMID: 18288995.

41. Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, et al. Puma is a dominant regulator of oxidative stress induced bax activation and neuronal apoptosis. J Neurosci. 2007; 27:12989–12999. PMID: 18032672.

42. van Heerde WL, Poort S, van't Veer C, Reutelingsperger CP, de Groot PG. Binding of recombinant annexin V to endothelial cells : effect of annexin V binding on endothelial-cell-mediated thrombin formation. Biochem J. 1994; 302(Pt 1):305–312. PMID: 8068019.

43. Vidavalur R, Otani H, Singal PK, Maulik N. Significance of wine and resveratrol in cardiovascular disease : French paradox revisited. Exp Clin Cardiol. 2006; 11:217–225. PMID: 18651034.
44. Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002; 958:439–447. PMID: 12470882.

45. Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet. 2003; 362:1211–1224. PMID: 14568745.

46. White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, et al. Brain ischemia and reperfusion : molecular mechanisms of neuronal injury. J Neurol Sci. 2000; 179(S 1-2):1–33. PMID: 11054482.
47. Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008; 15:1–14. PMID: 18220759.

48. Yılmaz MB, Tönge M, Emmez H, Kaymaz F, Kaymaz M. Neuroprotective effects of quetiapine on neuronal apoptosis following experimental transient focal cerebral ischemia in rats. J Korean Neurosurg Soc. 2013; 54:1–7. PMID: 24044072.

49. Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain : role of resveratrol in microglial activation. Eur J Pharmacol. 2010; 636:1–7. PMID: 20361959.

50. Zheng JP, Ju D, Jiang H, Shen J, Yang M, Li L. Resveratrol induces p53 and suppresses myocardin-mediated vascular smooth muscle cell differentiation. Toxicol Lett. 2010; 199:115–122. PMID: 20797428.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download