Abstract
We report a case of a 31-year-old woman with glioblastoma multiforme (GBM) in the pineal region with associated leptomeningeal dissemination and lumbar metastasis. The patient presented with severe headache and vomiting. Magnetic resonance imaging (MRI) of the brain showed a heterogeneously enhanced tumor in the pineal region with obstructive hydrocephalus. After an urgent ventricular-peritoneal shunt, she was treated by subtotal resection and chemotherapy concomitant with radiotherapy. Two months after surgery, MRI showed no changes in the residual tumor but leptomeningeal dissemination surrounding the brainstem. One month later, she exhibited severe lumbago and bilateral leg pain. Thoracico-lumbar MRI showed drop like metastasis in the lumbar region. Finally she died five months after the initial diagnosis. Neurosurgeons should pay attention to GBM in the pineal region, not only as an important differential diagnosis among the pineal tumors, but due to the aggressive features of leptomeningeal dissemination and spinal metastasis.
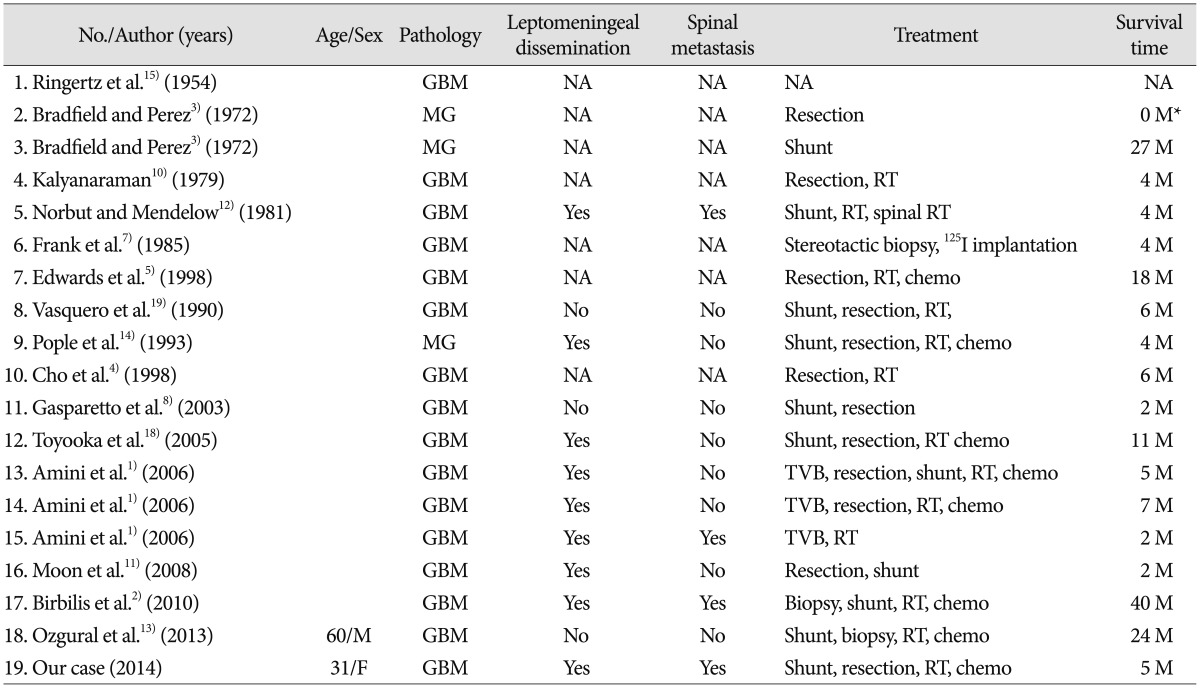
Glioblastoma multiforme (GBM) is the most common primary malignant tumor in the brain. Even though the best treatment involves maximal surgical resection followed by adjuvant radiotherapy and chemotherapy, the median survival time is less than two years17). GBM often occurs in the cerebral hemisphere, i.e., the frontal, temporal, and parietal lobes21). By contrast, the pineal region is a rare location for GBM. To our knowledge, only eighteen cases of GBM in the pineal region have been reported12345781011121314151819). In this case report, we aim to discuss the characteristics of GBM in the pineal region, and particularly the feature of spreading into the cerebro-spinal fluid space.
A 31-year-old woman with no previous history presented with persistent severe headache and repeated vomiting at a local clinic. MRI of the head revealed a pineal tumor with obstructive hydrocephalus. She was eventually transferred to our hospital for further treatment. She underwent an urgent ventricular-peritoneal shunt. After the ventricular-peritoneal shunt, she improved dramatically. Subsequently, gadolinium enhanced-MRI of the brain showed heterogeneous enhancement in the pineal region without pituitary tumor and leptomeningeal and ventricular dissemination (Fig. 1). Blood examination showed normal serum levels of α-fetoprotein, β human chronic gonadotrophin and placental alkaline phosphatase. Cerebral angiography revealed a vascular rich tumor fed by the posterior choroidal artery and arterial-venous shunting. In order to confirm the pathological diagnosis and remove the tumor, she underwent subtotal resection of the pineal tumor with craniotomy using the occipital transtentorial approach. In the intraoperative findings, the tumor was soft and bled easily. It was easy to dissect the tumor from the splenium. We concluded that the tumor originated in the pineal region, not in the splenium or midbrain. Finally, we performed subtotal resection because it was very difficult to stop the bleeding in the left superior and lateral parts of the tumor. She exhibited no new neurological deficits after the surgical resection.
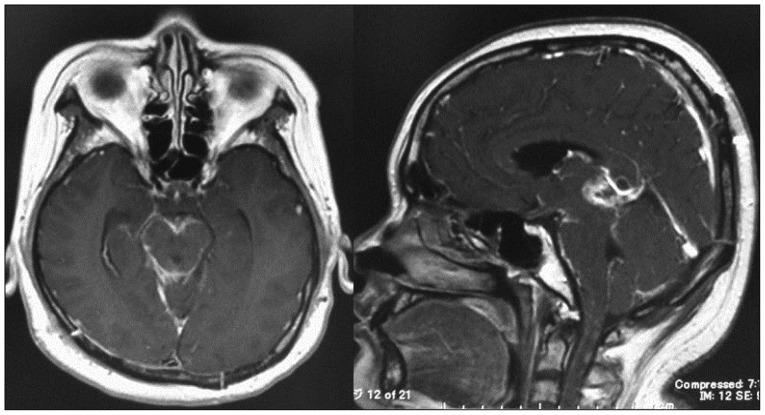
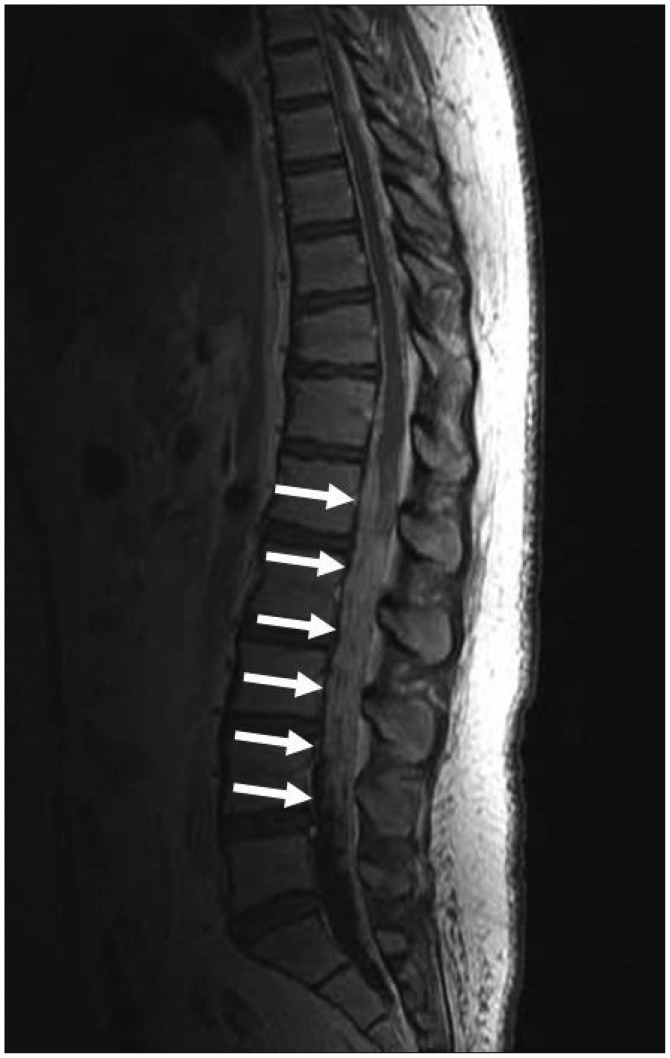
Histopathological findings showed the feature of glioblastoma composed of poorly differentiated pleomorphic tumor cells with marked nuclear atypia and brisk mitotic activity (Fig. 2). Focal necrosis with pseudopalisading was found, and MIB-1 proliferation index was high (43.7%). Immunohistochemistry showed a positive reaction to the glial fibrillary acidic protein, S-100, nestin, and INI-1. On the other hand, it showed a negative reaction to synaptophysin and NFP-MH. After surgical resection, she was treated by radiotherapy with 60 Gy and daily administration temozolomide (75 mg/m2) for forty-two days. Two months later, postoperative MRI showed no change of the residual tumor but leptomeningeal dissemination surrounding the brain stem and upper cervical spinal cord (Fig. 3). She continued with temozolomide chemotherapy. Three months after surgery, she experienced severe lumbago and bilateral leg pain. Thoracico-lumbar MRI showed drop metastasis (Fig. 4). She and her family refused any additional treatment, and finally she died five months after the initial diagnosis.
GBM is categorized as grade 4 in the WHO scale, and is the most common malignant tumor in the brain, and often occurs in the cerebral hemisphere in adults21). On the other hand, the pineal region is a rare location for GBM. GBM in the pineal region was reported for the first time in 1954 by Ringertz et al.15). Since then, to our knowledge, only eighteen cases of GBM in the pineal region have been reported12345781011121314151819). The presenting symptoms of GBM in the pineal region are related to obstructive hydrocephalus, such as headache, nausea and vomiting, visual disturbance. Indeed, in many of the reported cases, including our case, ventricular-peritoneal shunts were required. Secondly, the symptoms are related to the direct compression to the midbrain, i.e., Parinaud's palsy.
A summary of glioblastoma multiforme in the pineal region is presented in the Table 1. Among the nineteen cases of pineal GBM reported previously including our case, eleven patients were women and seven were men. The mean age was 42.5 years old (from 5 to 68 years). While twelve out of all cases were mentioned concerning the leptomeningeal dissemination, nine (75%) of them exhibited leptomeningeal dissemination upon autopsy or radiological findings using computed tomography (CT) and MRI. Concerning the spinal metastasis, four cases (25%) exhibited spinal metastasis; in the lumbosacral region in two, in the lower cervical and upper thoracic region in one, in the diffuse leptomeningeal carcinomatosis in one. In our review of GBMs in the pineal region, four spinal metastases (25%) developed out of eleven cases of pineal GBM. On the other hand, among the common GBMs, only 1.1% of all GBMs developed spinal metastases16). Symptomatic spinal metastases from GBM are rarely reported because most patients would not survive for the long time9). Furthermore, the interval between the diagnosis of the spinal metastasis and death was 2 to 3 months20). This insight suggested that the site of pineal region may play an important role in the development of spinal GBM metastasis and in the poor prognosis ofxe pineal GBM.
It remains controversial as to precisely when the spinal metastasis occurs. Birbilis et al.2) pointed out the potential for increased spinal spread of tumor cells during craniotomy. On the other hand, Elliot et al.6) reported that the entry into the ventricle during the craniotomy did not significantly influence either CSF tumor dissemination or survival time in the supratentorial GBM. While the mechanism of spinal metastasis, at least, remains controversial, the location of GBM, including the pineal region, intraventricular region, and adjacent to the ventricle, appears to be an important factor of the dissemination to the CSF.
The review of GBMs located in the pineal region indicated the poor prognosis and tendency for metastasis to the CSF, compared with common GBM. Neurosurgeons should pay attention to GBMs in the pineal region, not only as an important differential diagnosis among the pineal tumors, but due to the aggressive features of leptomeningeal dissemination and spinal metastasis.
References
1. Amini A, Schmidt RH, Salzman KL, Chin SS, Couldwell WT. Glioblastoma multiforme of the pineal region. J Neurooncol. 2006; 79:307–314. PMID: 16645719.

2. Birbilis TA, Matis GK, Eleftheriadis SG, Theodoropoulou EN, Sivridis E. Spinal metastasis of glioblastoma multiforme : an uncommon suspect? Spine(Phila Pa 1976). 2010; 35:E264–E269. PMID: 20195200.
3. Bradfield JS, Perez CA. Pineal tumors and ectopic pinealomas. Analysis of treatment and failures. Radiology. 1972; 103:399–406. PMID: 4537183.
4. Cho BK, Wang KC, Nam DH, Kim DG, Jung HW, Kim HJ, et al. Pineal tumors : experience with 48 cases over 10 years. Childs Nerv Syst. 1998; 14:53–58. PMID: 9548342.
5. Edwards MS, Hudgins RJ, Wilson CB, Levin VA, Wara WM. Pineal region tumors in children. J Neurosurg. 1988; 68:689–697. PMID: 2451717.

6. Elliott JP, Keles GE, Waite M, Temkin N, Berger MS. Ventricular entry during resection of malignant gliomas : effect on intracranial cerebrospinal fluid tumor dissemination. J Neurosurg. 1994; 80:834–839. PMID: 8169622.

7. Frank F, Gaist G, Piazza G, Ricci RF, Sturiale C, Galassi E. Stereotaxic biopsy and radioactive implantation for interstitial therapy of tumors of the pineal region. Surg Neurol. 1985; 23:275–280. PMID: 3883555.

8. Gasparetto EL, Warszawiak D, Adam GP, Bleggi-Torres LF, de Carvalho Neto A. Glioblastoma multiforme of the pineal region : case report. Arq Neuropsiquiatr. 2003; 61:468–472. PMID: 12894287.
9. Han SR, Yee GT, Lee DJ, Whang CJ. Spinal metastases from supratentorial glioblastoma. J Korean Neurosurg Soc. 2005; 38:475–477.
10. Kalyanaraman UP. Primary glioblastoma of the pineal gland. Arch Neurol. 1979; 36:717–718. PMID: 508133.

11. Moon KS, Jung S, Jung TY, Kim IY, Lee MC, Lee KH. Primary glioblastoma in the pineal region : a case report and review of the literature. J Med Case Rep. 2008; 2:288. PMID: 18752674.
12. Norbut AM, Mendelow H. Primary glioblastoma multiforme of the pineal region with leptomeningeal metastases : a case report. Cancer. 1981; 47:592–596. PMID: 6261912.

13. Ozgural O, Kahilogullari G, Bozkurt M, Heper AO, Savas A. Primary pineal glioblastoma : a case report. Turk Neurosurg. 2013; 23:572–574. PMID: 24101287.
14. Pople IK, Arango JC, Scaravilli F. Intrinsic malignant glioma of the pineal gland. Childs Nerv Syst. 1993; 9:422–424. PMID: 8306360.

15. Ringertz N, Nordenstam H, Flyger G. Tumors of the pineal region. J Neuropathol Exp Neurol. 1954; 13:540–561. PMID: 13201970.

16. Stark AM, Nabavi A, Mehdorn HM, Blömer U. Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg Neurol. 2005; 63:162–169. discussion 169PMID: 15680662.

17. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.

18. Toyooka T, Miyazawa T, Fukui S, Otani N, Nawashiro H, Shima K. Central neurogenic hyperventilation in a conscious man with CSF dissemination from a pineal glioblastoma. J Clin Neurosci. 2005; 12:834–837. PMID: 16198924.

19. Vaquero J, Ramiro J, Martinez R. Glioblastoma multiforme of the pineal region. J Neurosurg Sci. 1990; 34:149–150. PMID: 1965444.
20. Vertosick FT Jr, Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme : a clinical series. Neurosurgery. 1990; 27:516–521. discussion 521-522PMID: 2172859.
21. Zada G, Bond AE, Wang YP, Giannotta SL, Deapen D. Incidence trends in the anatomic location of primary malignant brain tumors in the United States : 1992-2006. World Neurosurg. 2012; 77:518–524. PMID: 22120376.

Fig. 1
Preoperative enhanced magnetic resonance images revealing a heterogenous enhancement of a pineal tumor with obstructive hydrocephalus.
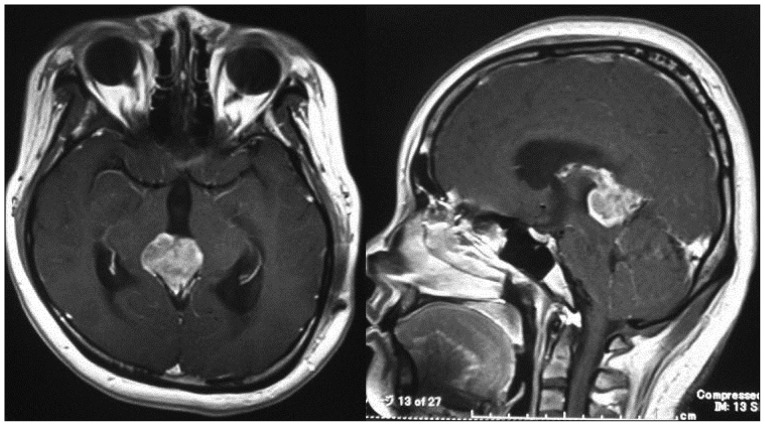
Fig. 2
Histopathological findings showing the feature of glioblastoma composed of poorly differentiated pleomorphic tumor cells with marked nuclear atypia and brisk mitotic activity and focal necrosis with pseudopalisading (H&E stain ×50).
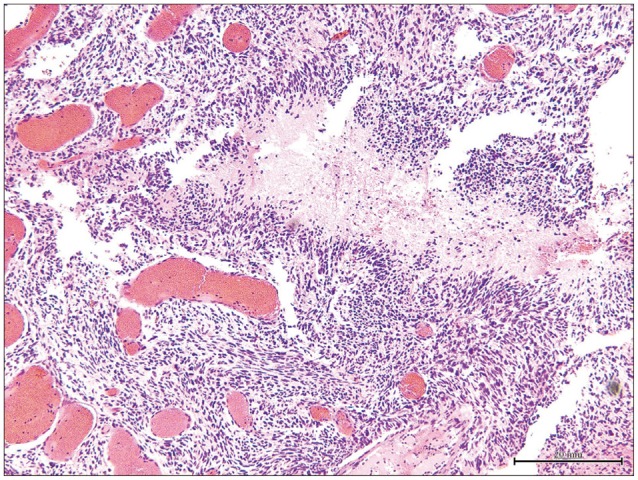
Fig. 3
Postoperative enhanced magnetic resonance images revealing an enhancement surrounding the brainstem and the residual tumor in the pineal region after radiotherapy concomitant chemotherapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download