Abstract
Objective
Pineal parenchymal tumors (PPTs) in adults are rare, and knowledge regarding their optimal management and treatment outcome is limited. Herein, we present the clinical results of our series of PPTs other than pineoblastomas managed by stereotactic radiosurgery (SRS) at upfront setting.
Methods
Between 1997 and 2014, nine consecutive adult patients with the diagnosis of PPTs, either pineocytoma or pineal parenchymal tumor of intermediate differentiation, were treated with SRS. There were 6 men and 3 women. The median age was 39 years (range, 31-53 years). All of the patients presented with symptoms of hydrocephalus. Endoscopic third ventriculostomy and biopsy was done for initial management. After histologic diagnosis, patients were treated with Gamma Knife with the mean dose of 13.3 Gy (n=3) or fractionated Cyberknife with 32 Gy (n=6).
Results
After a mean follow-up of 78.6 months (range, 14-223 months), all patients were alive and all of their tumors were locally controlled except for one instance of cerebrospinal fluid seeding metastasis. On magnetic resonance images, tumor size decreased in all patients, resulting in complete response in 3 patients and partial response in 6. One patient had experienced temporary memory impairment after SRS, which improved spontaneously.
Tumors involving the pineal region are rare and challenging for both diagnosis and management. Among those, pineal parenchymal tumors (PPTs) account for 15% to 30% and other tumors in this region include germ cell tumors of variable subtypes, gliomas, and metastases19). To provide an optimal therapy for each specific type of these tumors, correct tissue diagnosis is essential. Simultaneously, strategies for restoration or diversion of cerebrospinal fluid (CSF) dynamics are required, because most of patients present with symptoms of obstructive hydrocephalus caused by the tumors.
Until recently, PPTs had been classified as either benign, indolent pineocytomas (PCs) of World Health Organization (WHO) grade 1 or malignant, aggressive pineoblastomas (PBs) of WHO grade 4. In 2007 WHO classification of tumors of the central nervous system, pineal parenchymal tumors of intermediate differentiation (PPTIDs) of WHO grade 2 or 3 were added to this classification, which are considered to occur within the spectrum of pathogenesis between PCs and PBs2127). Of these, PBs comprise 25% to 50% of PPTs and are most commonly seen in pediatric patients. As the biological behavior of PBs is highly malignant, they are managed with aggressive multimodal approaches combining surgery, radiotherapy (RT) and chemotherapy1028). On the other hand, consensus on the management of PPTs in adults, mostly consisting of PCs and PPTIDs, is limited to date, primarily because of their rarity. Complete surgical resection can provide a chance of cure for PCs830). However, it is not always feasible and the risks of morbidity and mortality should be carefully balanced3413). Furthermore, it is still unknown whether or not complete resection could provide a cure for PPTIDs, as PPTIDs of higher histologic grade are potentially more aggressive than PCs. In this background, stereotactic radiosurgery (SRS) can be considered as a safe alternative or adjunct to surgery for local tumor control (LTC) and has been reportedly effective and safe in treating PPTs162025263031). Since 1997, we elected SRS as a primary therapy following CSF diversion and biopsy for PPTs other than PBs in adults, and nine consecutive patients have been treated accordingly.
This retrospective study was approved by our institutional review board. Between 1997 and 2014, nine consecutive adult patients with PPTs other than PBs were treated with SRS at upfront setting. There were 3 patients with histologic diagnosis of PCs, 5 with PPTIDs, and one with only clinical diagnosis (described elsewhere in this section). All the medical records and neuroimages of the patients were analyzed.
There were 6 men and 3 women. The median age of the patients was 39 years (range, 31-53 years). All patients presented with symptoms of obstructive hydrocephalus caused by the tumor, including headache in 6 patients, diplopia in 2, and gait disturbance and dizziness in each one patient. Papilledema was seen in 4 of 5 patients with available fundoscopic examination data. Table 1 summarizes the clinical data of the patients.
On magnetic resonance imaging (MRI), tumors represented low signal intensity on T1-weighted images and high signal intensity on T2-weighted images, and enhanced well heterogeneously (5 of 9) or homogeneously (4 of 9) with gadolinium. Five tumors were irregular in shape and had a fuzzy margin, suggesting suspicious infiltration into surrounding structures (3 PPTIDs, 1 PC, and 1 unspecified). The median tumor volume was 7.7 cc (range, 3.6-13.4 cc). Obstructive hydrocephalus was observed in all patients. No evidence of CSF seeding was seen on initial MRI. On computed tomography (CT), tumors were isodense or slightly hyperdense. Intratumoral calcification was noted in one patient.
For initial management, 8 of the 9 patients underwent the endoscopic third ventriculostomy (ETV) and biopsy of the tumor. One patient (Case No. 2) had been referred from another hospital after a craniotomy and biopsy with placement of a ventriculoperitoneal shunt. With the initial endoscopic surgery, patient symptoms were relieved immediately and tumor tissue was obtained successfully in all but one patient (Case No. 9; biopsy failed owing to massive intraoperative bleeding). This patient refused a second operation and was treated empirically with platinum-based chemotherapy, because the incidence of germinoma is highest in this location of tumor in Far Eastern Asian population. However, the tumor did not respond at all to the chemotherapy, and treatment was switched to SRS, as PPTs were considered as a second possible diagnosis.
PCs are primarily composed of well-differentiated cells with resemblance to mature pineocytes. Pineocytomatous rosettes are frequently observed in PCs. On the contrary, PPTIDs show moderate nuclear atypia and higher mitotic counts than PCs. Pineocytomatous rosettes are inconspicuous in PPTIDs, consistent with partial loss of differentiation11). In reviewing 8 cases with histologic diagnosis of PPTs, PCs represented sparsely cellular tumors composed of monotonous small round cells, even smaller than normal parenchymal cells of the pineal gland. Pineocytomatous rosettes composed of acellular areas of neuropils surrounded by small round tumor cells were observed. No mitoses were seen. PPTIDs were of moderate or high cellularity with some tumors showing tightly compacted tumor cells while others being less cellular and lobulated. Tumor cells exhibited more or less nuclear pleomorphism. Considerable numbers of bizarre giant tumor cells were mixed, but mitoses were rare and the proliferative activity measured by MIB-1 index was low (<5%). Necrosis was not observed. Tumor cells were diffusely immunoreactive for synaptophysin.
Gamma knife (GK) (Elekta AB, Stockholm, Sweden) was performed on patients during the early period (Case No. 1 to 3). Since 2011, Cyberknife (CK) (Accuray Inc., Sunnyvale, CA, USA) has been used to treat patients with the intention of fractionation delivery of SRS to minimize radiation toxicity to adjacent critical structures. For GK treatment, the mean marginal dose of 13.3 Gy (range, 12-16 Gy) was prescribed to 50% isodose lines, and for fractionated CK, the mean marginal dose of 32 Gy (range, 30-36 Gy) was prescribed to the mean 80% isodose line (range, 77-82%) and delivered in 5 daily fractions.
Clinical examinations and MRI scans were performed at 6-months intervals during the first year, annually for two years, and biennially thereafter. Treatment responses were divided into four categories according to the criteria of Macdonald et al.24) : complete response, partial response (>50% reduction in size), progressive disease (>25% increase in size), and stable disease. LTC was defined as complete or partial response and stable disease.
After a mean follow-up of 78.6 months (range, 14-223 months), all patients were alive and all of their tumors were locally controlled except for one instance of CSF seeding metastasis (Case No. 1). On MRI, tumor size decreased in all patients, resulting in complete response in 3 patients and partial response in 6. Treatment-related adverse event was observed in one patient who had experienced temporary memory impairment one year after SRS, which improved spontaneously (Case No. 5).
A 34-year-old woman presented with a one-month history of headache. Papilledema was observed on fundoscopic examination. MRI revealed a 3.2×2.4×2.0 cm sized tumor in the pineal region with obstructive hydrocephalus (Fig. 1A). The tumor was irregular in shape and appeared to infiltrate into surrounding structures. After ETV and biopsy, her headache was relieved and histologic diagnosis proved to be PPTID (Fig. 1B-E). She received fractionated CK treatment with marginal dose of 30 Gy one month after surgery (Fig. 1F). A marked decrease in tumor size was observed in 3 months after CK and tumor disappeared completely in 9 months. No evidence of disease was seen at the last follow-up of 3 years (Fig. 1G).
A 44-year-old man was referred for pineal region tumor. He previously underwent a craniotomy and biopsy of the tumor with ventriculoperitoneal shunt placement at another hospital. On MRI, a 2.8×1.8×2.2 cm sized tumor was observed in the pineal region (Fig. 2A), and histologic examination confirmed the diagnosis of PC (Fig. 2B). After GK treatment with marginal dose of 12 Gy, he achieved durable tumor response with more than 18 years of follow-up (Fig. 2C).
A 31-year-old man presented with a 4-month history of diplopia. MRI revealed a 1.4×1.8×3 cm sized tumor in the pineal and tectal region with obstructive hydrocephalus (Fig. 3A). After ETV and biopsy, PPTID was diagnosed. He received GK treatment with marginal dose of 16 Gy. Complete tumor response was achieved and the primary site remained free of disease, until remote CSF seeding metastases were found in the ventricles and the cauda equina 7 years after GK (Fig. 3B, C). He received additional craniospinal irradiation and his disease was stable at 17 years.
Pineal region tumors, although rare, consist of variable histologic entities including germ cell tumors most frequently followed by PPTs, gliomas, and metastases. To make a differential diagnosis among these entities, multiple factors, such as patient age, presenting symptoms, imaging features, and serum/CSF biochemical markers, are considered and may help in predicting tumor type. Those factors, however, are not characteristic to a certain specific histologic entity. As different treatment strategies are required according to the histologic diagnosis, correct tissue diagnosis is essential to provide an optimal therapy. Together with this, as a majority of patients with tumors in this region present with symptoms of hydrocephalus, simultaneous ETV and tumor biopsy frequently offers an initial management of choice, obviating the risks of unnecessary craniotomy and permanent shunt placement. All of our patients were managed in this way except for one referred from another hospital.
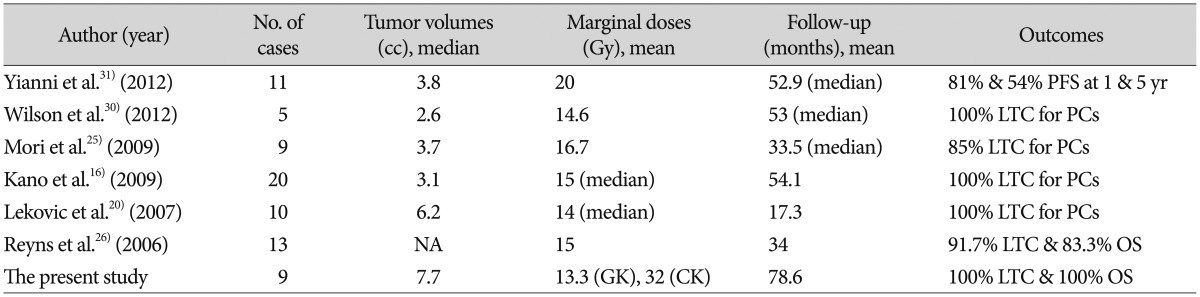
Apart from PBs which are common in pediatric patients and are managed by aggressive multimodal approaches, optimal management of non-malignant PPTs in adults remains an issue of debate. For benign PCs, attempting an aggressive total resection of tumor is justifiable with an enhanced chance for cure compared with incomplete resection with or without adjuvant RT, which is supported by a recent systematic review of the literature7). Although radical surgery via the supracerebellar infratentorial or the occipital transtentorial routes has been advocated based on this grounds, complete tumor resection was achieved only in a half of patients45618), and perioperative mortality and permanent morbidity have been reported in 0-11% and 19-28%, respectively1518192229). Thus, decision-making on aggressive surgery should be carefully balanced against the potentially life-threatening risks. In case of incomplete resection or biopsy, adjuvant fractionated RT is usually employed, but it appears to provide no benefit in overall survival as well as LTC for this radioresistant, indolent subtype of PPTs8). Alternatively, SRS is used increasingly to treat PPTs. Recent studies reported excellent long-term LTC and survival in patients with PPTs, especially with PCs after SRS, mostly at adjuvant or salvage settings and occasionally at upfront setting (Table 2)12162025263031), suggesting SRS may be a useful alternative or adjunct to surgical resection. In extension from these observations, we elected SRS as a primary therapeutic modality after histologic confirmation of PPTs other than PBs and achieved LTC and survival in all patients except for one instance of CSF seeding metastasis. All those previous studies including our own demonstrated a great safety of SRS without any treatment-related permanent morbidity or mortality.
One unique characteristic of our series is incorporation of many cases diagnosed with PPTIDs. The biological behavior of PPTIDs of higher WHO grade is generally considered less predictable than that of PCs, and sometimes exhibits an aggressive nature27). The 5-year survival rates have been estimated at 74% for WHO grade 2 PPTIDs and 39% for grade 3 PPTIDs9). Recurrence rates are reportedly also higher in grade 3 PPTIDs. Along with these, local invasion of PPTIDs was frequently observed on MRI17), which was similar in our series. Despite all those relatively dismal features of PPTIDs compared to PCs, these tumors responded well to SRS as PCs, yielding 100% rates of LTC and overall survival in our study. Yet, remote CSF metastases occurred in one patient 7 years after SRS. Currently, questions regarding the optimal management of PPTIDs are largely unanswered including which treatment option would be most beneficial among surgical resection, RT, SRS, and chemotherapy and whether a combination of therapy would be better or not, although these tumors have been reported to be radiosensitive as seen in our cases21423). Further studies are needed to elucidate the pathogenesis, biological behavior, and an optimal therapy for this particular kind of new entity.
Simultaneous tissue diagnosis and CSF diversion can be achieved safely using ETV and biopsy as an initial management for pineal region tumors causing obstructive hydrocephalus. For PCs and PPTIDs, the frequent subtypes of PPTs in adults, SRS appears to be effective and safe and can be considered as a useful alternative or adjuvant option to surgical resection.
Acknowledgements
This paper was presented at the 24th annual meeting of the Korean Brain Tumor Society on June 28, 2014.
References
1. Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I. Pineal gland tumors : experience from the SEER database. J Neurooncol. 2009; 94:351–358. PMID: 19373436.

2. Anan M, Ishii K, Nakamura T, Yamashita M, Katayama S, Sainoo M, et al. Postoperative adjuvant treatment for pineal parenchymal tumour of intermediate differentiation. J Clin Neurosci. 2006; 13:965–968. PMID: 16904896.

3. Blakeley JO, Grossman SA. Management of pineal region tumors. Curr Treat Options Oncol. 2006; 7:505–516.

4. Bruce JN, Ogden AT. Surgical strategies for treating patients with pineal region tumors. J Neurooncol. 2004; 69:221–236. PMID: 15527093.

5. Bruce JN, Stein BM. Surgical management of pineal region tumors. Acta Neurochir (Wien). 1995; 134:130–135. PMID: 8748771.

6. Chandy MJ, Damaraju SC. Benign tumours of the pineal region : a prospective study from 1983 to 1997. Br J Neurosurg. 1998; 12:228–233. PMID: 11013685.
7. Clark AJ, Ivan ME, Sughrue ME, Yang I, Aranda D, Han SJ, et al. Tumor control after surgery and radiotherapy for pineocytoma. J Neurosurg. 2010; 113:319–324. PMID: 20136388.

8. Clark AJ, Sughrue ME, Aranda D, Parsa AT. Contemporary management of pineocytoma. Neurosurg Clin N Am. 2011; 22:403–407. PMID: 21801989.

9. Fauchon F, Jouvet A, Paquis P, Saint-Pierre G, Mottolese C, Ben Hassel M, et al. Parenchymal pineal tumors : a clinicopathological study of 76 cases. Int J Radiat Oncol Biol Phys. 2000; 46:959–968. PMID: 10705018.
10. Fontana EJ, Garvin J, Feldstein N, Anderson RC. Pediatric considerations for pineal tumor management. Neurosurg Clin N Am. 2011; 22:395–402. PMID: 21801988.

11. Han SJ, Clark AJ, Ivan ME, Parsa AT, Perry A. Pathology of pineal parenchymal tumors. Neurosurg Clin N Am. 2011; 22:335–340. PMID: 21801981.

12. Hasegawa T, Kondziolka D, Hadjipanayis CG, Flickinger JC, Lunsford LD. The role of radiosurgery for the treatment of pineal parenchymal tumors. Neurosurgery. 2002; 51:880–889. PMID: 12234394.

13. Hernesniemi J, Romani R, Albayrak BS, Lehto H, Dashti R, Ramsey C 3rd, et al. Microsurgical management of pineal region lesions : personal experience with 119 patients. Surg Neurol. 2008; 70:576–583. PMID: 19055952.

14. Ito T, Kanno H, Sato K, Oikawa M, Ozaki Y, Nakamura H, et al. Clinicopathologic study of pineal parenchymal tumors of intermediate differentiation. World Neurosurg. 2014; 81:783–789. PMID: 23396072.

15. Kang JK, Jeun SS, Hong YK, Park CK, Son BC, Lee IW, et al. Experience with pineal region tumors. Childs Nerv Syst. 1998; 14:63–68. PMID: 9548344.

16. Kano H, Niranjan A, Kondziolka D, Flickinger JC, Lunsford D. Role of stereotactic radiosurgery in the management of pineal parenchymal tumors. Prog Neurol Surg. 2009; 23:44–58. PMID: 19329860.

17. Komakula S, Warmuth-Metz M, Hildenbrand P, Loevner L, Hewlett R, Salzman K, et al. Pineal parenchymal tumor of intermediate differentiation : imaging spectrum of an unusual tumor in 11 cases. Neuroradiology. 2011; 53:577–584. PMID: 21080159.

18. Konovalov AN, Pitskhelauri DI. Principles of treatment of the pineal region tumors. Surg Neurol. 2003; 59:250–268. PMID: 12748006.

19. Lapras C, Patet JD, Mottolese C, Lapras C Jr. Direct surgery for pineal tumors : occipital-transtentorial approach. Prog Exp Tumor Res. 1987; 30:268–280. PMID: 3628812.
20. Lekovic GP, Gonzalez LF, Shetter AG, Porter RW, Smith KA, Brachman D, et al. Role of Gamma Knife surgery in the management of pineal region tumors. Neurosurg Focus. 2007; 23:E12. PMID: 18081477.

21. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109. PMID: 17618441.

22. Luo SQ, Li DZ, Zhang MZ, Wang ZC. Occipital transtentorial approach for removal of pineal region tumors : report of 64 consecutive cases. Surg Neurol. 1989; 32:36–39. PMID: 2734686.

23. Lutterbach J, Fauchon F, Schild SE, Chang SM, Pagenstecher A, Volk B, et al. Malignant pineal parenchymal tumors in adult patients : patterns of care and prognostic factors. Neurosurgery. 2002; 51:44–55. discussion 55-56PMID: 12182434.
24. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280. PMID: 2358840.

25. Mori Y, Kobayashi T, Hasegawa T, Yoshida K, Kida Y. Stereotactic radiosurgery for pineal and related tumors. Prog Neurol Surg. 2009; 23:106–118. PMID: 19329865.

26. Reyns N, Hayashi M, Chinot O, Manera L, Péragut JC, Blond S, et al. The role of Gamma Knife radiosurgery in the treatment of pineal parenchymal tumours. Acta Neurochir (Wien). 2006; 148:5–11. discussion 11PMID: 16172830.

27. Sato K, Kubota T. Pathology of pineal parenchymal tumors. Prog Neurol Surg. 2009; 23:12–25. PMID: 19329858.

28. Selvanathan SK, Hammouche S, Smethurst W, Salminen HJ, Jenkinson MD. Outcome and prognostic features in adult pineoblastomas : analysis of cases from the SEER database. Acta Neurochir (Wien). 2012; 154:863–869. PMID: 22460262.

29. Vaquero J, Ramiro J, Martínez R, Bravo G. Neurosurgical experience with tumours of the pineal region at Clinica Puerta de Hierro. Acta Neurochir (Wien). 1992; 116:23–32. PMID: 1319669.

30. Wilson DA, Awad AW, Brachman D, Coons SW, McBride H, Youssef E, et al. Long-term radiosurgical control of subtotally resected adult pineocytomas. J Neurosurg. 2012; 117:212–217. PMID: 22702479.

31. Yianni J, Rowe J, Khandanpour N, Nagy G, Hoggard N, Radatz M, et al. Stereotactic radiosurgery for pineal tumours. Br J Neurosurg. 2012; 26:361–366. PMID: 22168969.

Fig. 1
A 34-year-old woman with pineal parenchymal tumor of intermediate differentiation (Case No. 7). A : Gadolinium-enhanced magnetic resonance images showing a 3.2×2.4×2.0 cm sized tumor in the pineal region with obstructive hydrocephalus. B : Tumor shows diffusely high cellularity and tumor cell nuclei are pleomorphic. C : Multinucleated giant tumor cells are frequently seen (arrows). D : Tumor cells are diffusely immunoreactive for synaptophysin. E : Proliferation activity assessed by MIB-1 is low. F : She received 5-fraction Cyberknife (CK) treatment with marginal dose of 30 Gy. G : Tumor disappeared completely in 9 months after CK, and no evidence of disease was seen at the last follow-up of 3 years.
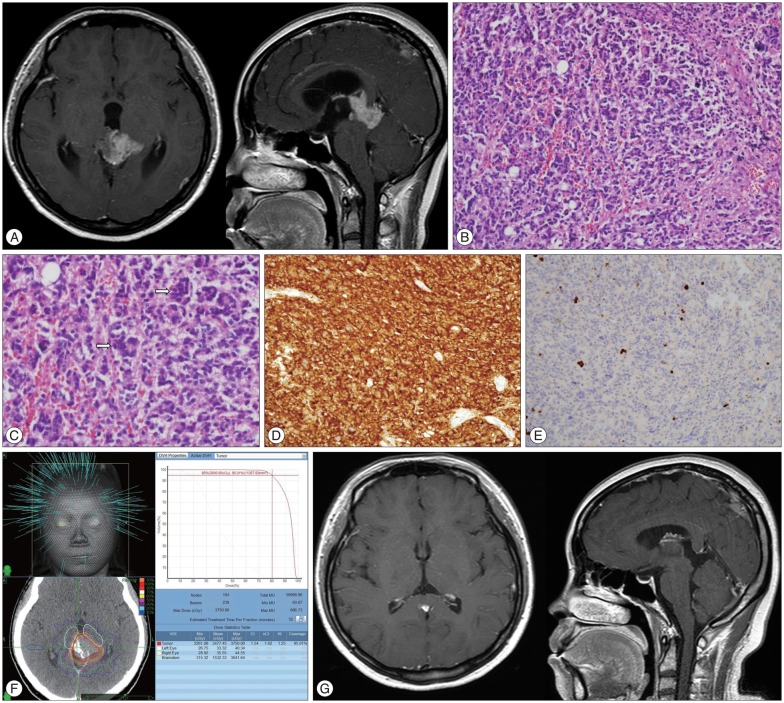
Fig. 2
A 44-year-old man with pineocytoma (Case No. 2). A : Gadolinium-enhanced magnetic resonance images at the time of Gamma Knife (GK) treatment. B : Tumor consists of relatively small, uniform, mature cells resembling normal pineocytes, and cell-free spaces filled with cell processes are forming vague rosettes. C : After GK treatment with marginal dose of 12 Gy, he achieved durable tumor response with more than 18 years of follow-up.
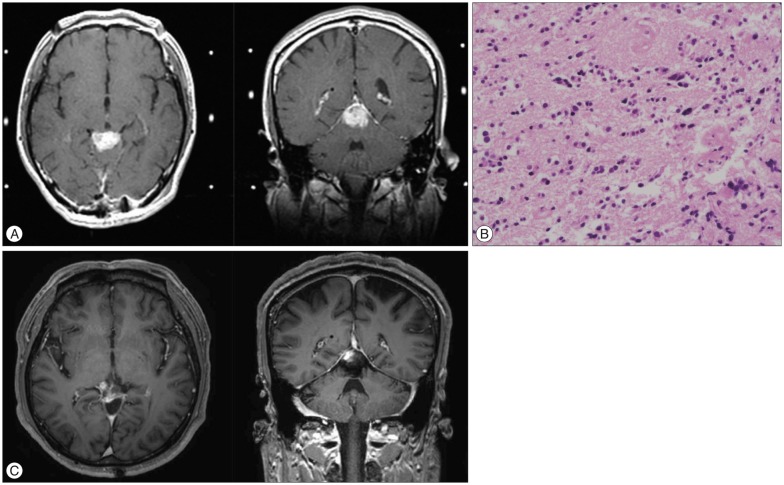
Fig. 3
A 31-year-old man with pineal parenchymal tumor of intermediate differentiation (Case No. 1). Gadolinium-enhanced magnetic resonance images before (A) and 84 months after Gamma Knife treatment (B). Although the primary site remained free of disease at this time, cerebrospinal fluid seeding metastases were observed in the ventricles and the cauda equina (C, arrows). He received additional craniospinal irradiation and his disease was stable at 17 years.
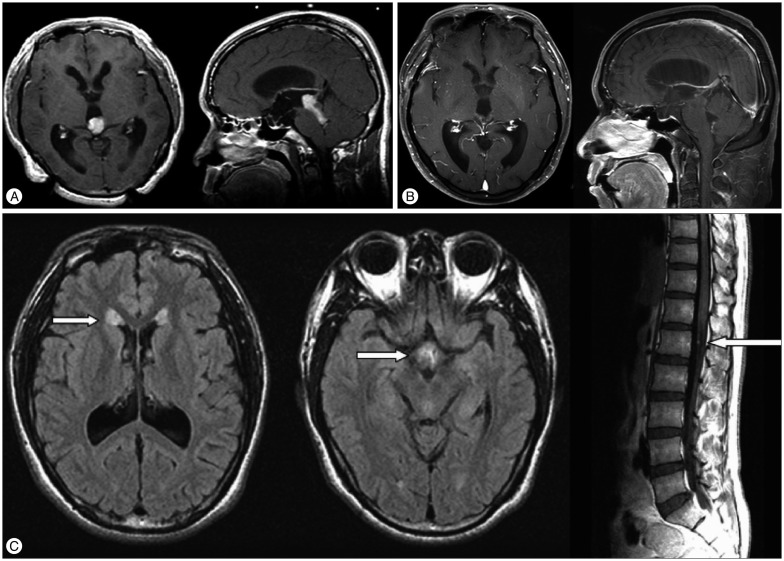
Table 1
Clinical summary of nine cases with pineal parenchymal tumors
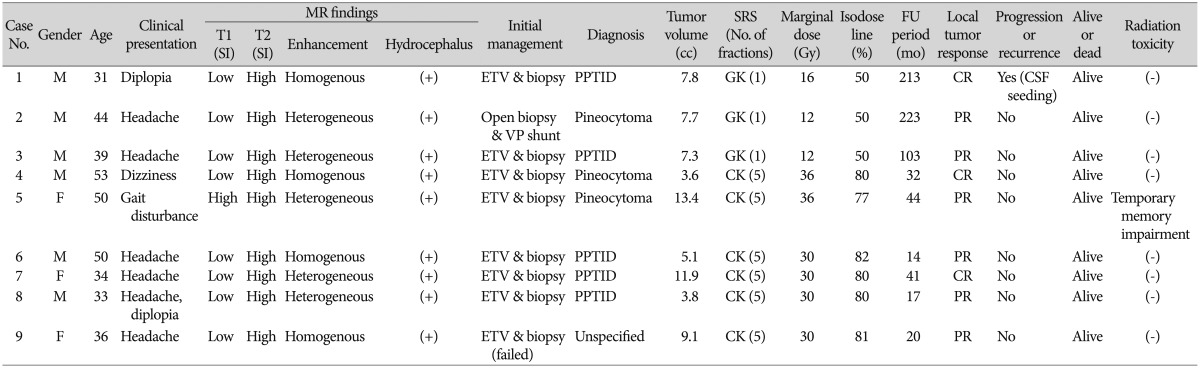
SI : signal intensity, SRS : stereotactic radiosurgery, FU : follow-up, M : male, F : female, ETV : endoscopic third ventriculostomy, PPRID : pineal parenchymal tumor of intermediate differentiation, GK : Gamma Knife, CR : complete response, CSF : cerebrospinal fluid, VP : ventriculoperitoneal, PR : partial response, CK : Cyberknife




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download