Abstract
Objective
To determine whether the use of contrast enhancement (especially its extent) predicts malignant brain edema after intra-arterial thrombectomy (IAT) in patients with acute ischemic stroke.
Methods
We reviewed the records of patients with acute ischemic stroke who underwent IAT for occlusion of the internal carotid artery or the middle cerebral artery between January 2012 and March 2015. To estimate the extent of contrast enhancement (CE), we used the contrast enhancement area ratio (CEAR)-i.e., the ratio of the CE to the area of the hemisphere, as noted on immediate non-enhanced brain computed tomography (NECT) post-IAT. Patients were categorized into two groups based on the CEAR values being either greater than or less than 0.2.
Results
A total of 39 patients were included. Contrast enhancement was found in 26 patients (66.7%). In this subgroup, the CEAR was greater than 0.2 in 7 patients (18%) and less than 0.2 in the other 19 patients (48.7%). On univariate analysis, both CEAR ≥0.2 and the presence of subarachnoid hemorrhage were significantly associated with progression to malignant brain edema (p<0.001 and p=0.004), but on multivariate analysis, only CEAR ≥0.2 showed a statistically significant association (p=0.019). In the group with CEAR ≥0.2, the time to malignant brain edema was shorter (p=0.039) than in the group with CEAR <0.2. Clinical functional outcomes, based on the modified Rankin scale, were also significantly worse in patients with CEAR ≥0.2 (p=0.003)
The main goal of treatment for patients with acute ischemic stroke is to salvage as much brain tissue as possible. Intravenous administration of a recombinant tissue plasminogen activator (IV tPA) such as alteplase has been considered an effective treatment option6). However, IV tPA has well-known limitations, such as its narrow window of therapeutic effectiveness and its many contraindications10), as well as its limited efficacy in the early recanalization of acute, large-vessel occlusions2). Because a good prognosis depends on rapid recanalization in acute ischemic stroke due to major vessel occlusion411), intra-arterial endovascular treatment is now considered a more reasonable option for treating ischemic stroke.
Previous randomized, controlled trials of intra-arterial approaches concluded that there were no significant differences between endovascular therapy and standard IV alteplase3512). However, two recently reported trials-the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN)1) and the Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE) trial8)-showed clinical benefits of endovascular treatments such as intra-arterial thrombectomy (IAT).
In our hospital, non-enhanced brain computed tomography (NECT) scans are obtained immediately after IAT to detect complications of the procedure, such as hemorrhage. Sometimes regions of high density could be seen on these scans and is believed to be evidence of contrast material used to enhance visualization during IAT. Its presence could disrupt the blood-brain barrier due to infarction16). Previous studies have suggested that this high density, referred to as "contrast enhancement," could be predictive of malignant brain edema or hemorrhagic transformations1421). After having witnessed several such cases in our hospital and on reviewing of previous studies, we hypothesized that a large area of contrast enhancement could signal malignant brain edema and thus a poor prognosis. Because the extent of enhancement as a predictive factor has rarely been reported, we conducted a retrospective study to test this hypothesis.
In this retrospective study we reviewed the database of patients who underwent IAT for acute occlusion of the internal cerebral artery (ICA) or the main trunk of the middle cerebral artery (MCA) from January 2012 through March 2015 in our institution. Patients were included in the analysis if they 1) had undergone emergency IAT and had been followed for longer than 3 months after the procedure, 2) had acute occlusion of the ICA or MCA due to thromboembolism, and 3) had undergone NECT scanning immediately after the emergency IAT. Patients were excluded if they had had acute occlusion of the anterior cerebral artery, acute occlusion of large arteries in the posterior circulation, or a history of IAT.
The following data were extracted from the medical records : age at the time of acute occlusion, sex, atrial fibrillation, hypertension, diabetes mellitus, a history of anticoagulant or antiplatelet therapy, National Institutes of Health Stroke Scale (NIHSS) score before and after IAT, the use of IV tPA, time to malignant brain edema after IAT, and modified Rankin scale (at 3 months after IAT. Malignant brain edema was defined as midline shifting of more than 5 mm of the affected hemisphere with compression of the brainstem on follow-up brain CT scans or brain magnetic resonance imaging (MRI) in patients with neurologic deterioration (defined as a decrease in Glasgow Coma Score (GCS) by more than 2 points or a 2-mm dilatation in pupil size as compared with the contralateral pupil).
The study protocol was approved by the Institutional Review Board of Cheongju St. Mary's Hospital at the Catholic University of Korea (No. IRB-98). Informed consent was not obtained because of the retrospective nature of the study.
The criteria for emergency IAT were as follows : 1) NIHSS score greater than 4, 2) no evidence of hemorrhage on brain CT scan, 3) low-density lesion covering less than one third of the MCA territory on brain CT scan, 4) no improvement in NIHSS score after administration of IV tPA within 4.5 hours of the start of treatment, 5) severe stenosis or occlusion of the ICA or MCA on digital subtraction cerebral angiography, or 6) poor collateral flow to the territory of the affected artery.
In our hospital, we put a premium on performing IAT as soon as possible in patients with an acute infarction due to occlusion of a major artery. Therefore, brain MRI is not routinely performed if the clinician confirms this diagnosis based on the results of brain CT scanning and neurologic examination.
Under local anesthesia all patients underwent digital subtraction angiography via a femoral artery. If severe stenosis or occlusion of the ICA or MCA was found and collateral flow to the affected area was poor, IAT was undertaken. Initially, an IV bolus of heparin, 3000 IU, was administered, followed by heparin, 1,000 IU, administered by injection every 60 minutes during the procedure. An 8 Fr or 9 Fr Cello balloon guide catheter (ev3 Neurovascular, Irvine, CA, USA) was advanced into the proximal ICA. IAT was performed using a stent clot retriever (Solitaire FR, ev3 Neurovascular, Irvine, CA, USA), or an aspiration device (Penumbra, Alameda, CA, USA).
NECT scans were taken immediately and 24 hours after IAT in all patients. Contrast enhancement was defined as hyperdense changes in natural anatomic shapes without mass effect that were observed on the initial NECT scans but not on the 24-hour follow-up scans.
It was impractical to take the time to determine Hounsfield units (HU) for the hyperdense changes because the purpose of this study was to rapidly determine factors that might predict progression to malignant brain edema. Instead, we compared the hyperdense changes seen on NECT scans with scans of the contralateral hemisphere.
The region of interest was marked on the NECT scans to show the area of contrast enhancement at the level of foramen of Monro and was used as an arbitrary standard for comparison. We thought images at the level of the foramen of Monro could be expected to show both the basal ganglia and the cortex area, which fully represented the vascular territory of the affected artery, and could constitute the standard level (Fig. 1). The contrast enhancement area was automatically calculated by means of picture archiving and communication system (PACS) software (Infinitt, Siemens, Erlangen, Germany). The preceding approach was also used to calculate the area of the hemisphere. The CEAR was the ratio of the contrast enhancement area to the area of the hemisphere, and patients were divided into two groups based on the CEAR values, either greater than 0.2 or less than 0.2, as calculated on logistic regression analysis.
A logistic regression analysis was used to determine the best value of CEAR for categorizing the patients into two groups. The chi-square test or Fisher's exact test was used in univariate analyses for categorical variables. The Mann-Whitney U test was used for continuous variables in non-parametric statistics. Multivariate analysis was performed using multiple logistic regression analysis to evaluate factors that might predict progression to malignant brain edema. Factors with a p value under 0.10 on univariate analyses were included in the multivariate analysis. All statistical analyses were done using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA), and a p value less than 0.05 was considered statistically significant.
A total of 39 patients were included in this study, with a mean (±SD) age of 70.5±13.03 years. The mean NIHSS score before IAT was 15.4±5.65. The ICA was occluded in 18 patients (46.1%). IV tPA was administered before IAT in 10 patients (25.6%), and 31 patients (79.5%) underwent IAT that involved a stent clot retriever. Successful recanalization on angiography was achieved in 34 patients (87.1%). Table 1 summarizes additional patient characteristics.
Contrast enhancement was observed in 26 patients (66.7%) and covered a mean area of 967.6±1217.43 sq mm. In 7 patients (18%) the CEAR was greater than 0.2. Subarachnoid hemorrhage and small intracranial hemorrhages were found in 12 patients (30.7%) and 2 patients (5.1%), respectively. More information is provided in Table 2.
Of all patients in this study, progression to malignant brain edema was in 10 patients (25.6%). On univariate analysis, a CEAR greater than 0.2 was significantly associated with progression to malignant brain edema (p<0.001). The presence of subarachnoid hemorrhage was also related to progression to malignant brain edema (p=0.004), but other factors failed to show a significant association. On multivariate analysis, only a CEAR greater than 0.2 was significantly associated with progression to malignant brain edema [odds ratio=20.626 (95% confidence interval=1.638-259.747); p=0.019]. More details are provided in Table 3, 4.
Patients with CEAR ≥0.2 on NECT scans obtained immediately after IAT showed rapid progression, as compared with the patients with CEAR <0.2. Time to malignant brain swelling in the group with CEAR ≥0.2 was 14.3±8.9 hours vs 33.7±11.02 hours in the group with CEAR <0.2 (p=0.039 on the Mann-Whitney U test) (Fig. 2). There was no significant difference between the two groups in mean modified Rankin scores prior to the acute infarction : 0.43±0.535 in the patients with CEAR ≥0.2 vs. 0.038±0.871 in those with CEAR <0.2 (p=0.363 on the Mann-Whitney U test). However, at 3 months after IAT, the modified Rankin scores for the group with CEAR ≥0.2 were significantly worse than those for the group with CEAR <0.2 (5.43±0.787 vs. 3.84±1.167, respectively) (p=0.003 on the Mann-Whitney U test) (Fig. 3).
In this study, NECT scans obtained immediately after IAT for acute occlusion of the ICA or MCA was found to be helpful in predicting the development of malignant brain edema and a subsequently poor prognosis. Patients with a CEAR greater than 0.2 were more likely to progress to malignant brain edema, and the edema worsened more rapidly.
It should be noted that the CEAR value of 0.2 in itself is not important because this value was calculated by dividing the area of the individual patient's hemisphere as a means of standardization and would most likely be different in different patients. The occlusion site in these patients with acute ischemic stroke was not only the MCA but also the ICA, so the area of the hemisphere became the denominator in the ratio. The more important point is that the extent of the contrast enhancement could be the predictive factor in this study-that is, the larger the area, the greater the risk for malignant brain edema and the poorer the prognosis.
After IAT, the main concerns are the patient's neurologic status, the extent of the infarcted area, and the possibility of progression to malignant brain swelling or hemorrhagic transformation. Previously, high NIHSS scores and low collateral blood supply was reported as a associated factor for malignant brain edema13).
After IAT, imaging studies, such as MRI, could help clinicians address these concerns. Nevertheless, performing MRI immediately in patients with acute ischemic stroke is difficult because it takes a long time and depends on the patient's clinical status. Thus, in our hospital, the protocol after IAT begins with obtaining an immediate NECT scan of the brain.
Our approach in identifying the area of contrast enhancement on NECT by comparing it with the contralateral hemisphere has both merits and demerits. One flaw is that this method is not absolute because a high-density area could reflect a small hemorrhage. In addition, some areas could be missed because this method depends on the examiner's ability to observe a density. Nevertheless, the advantages of this method include being able to detect possible malignant brain edema more quickly and providing intuitive information that will allow us decide on how to manage the patient with an acute ischemic stroke. Therefore, this method could be very useful for identifying areas of contrast enhancement on NECT scans obtained during the immediate post-IAT period. Reports of the rate at which contrast enhancement shows up on NECT after IAT vary greatly, from 22.6% to 84.2%141516171921); in our study, this rate was 66.7%. The reason for such wide variation may be differences in the definition of contrast enhancement, the number of patients, and the quality of the imaging equipment. In previous studies, the results concerning the presence of contrast enhancement and poor clinical outcomes have been controversial. After the first report by Wildenhain et al.20) in 1994, Nakano et al.17) and Yoon et al.21) documented an association between high-density areas found on NECT after IAT and a poor prognosis. However, several more recent studies have proposed that CE is of no value in predicting clinical outcome151619). In 2015, Kim et al.14) suggested that cortical involvement in contrast accumulation is associated with symptomatic hemorrhage and poor neurological outcomes. In our study, we found that the use of contrast enhancement by itself does not have statistical significance with respect to a poor clinical outcome, but rather the extent of enhancement is the predictive factor associated with poor prognosis.
According to a previous study, the presence of contrast enhancement on NECT scans obtained immediately after IAT is due to disruption of the blood-brain barrier. The suggested possible causes of this disintegration are initial ischemic injury, a toxic effect of the contrast media, and reperfusion injury. Ischemic damage to the microvasculature results in fragility of the basal lamina, which would normally prevent the cellular blood elements from extravasating out of the microvessels21). Contrast media is neurotoxic, and it has been hypothesized that the underlying mechanisms are hyperosmolality, increased pinocytosis, and the inherent chemotoxicity of these agents16). Finally, reperfusion injury may damage the blood-brain barrier because of its known association with leukocyte, platelet, complement, and hyperperfusion18). A larger area of contrast enhancement may mean more widespread disruption of the blood-brain barrier and an inevitably poor outcome, such as malignant brain edema.
Taken together, the findings suggest that contrast enhancement on NECT scans obtained immediately after IAT could represent a range of reperfusion injuries. Another notable finding in this study is that patients with CEAR greater than 0.2 progressed more rapidly to malignant brain edema. It is thought that reperfusion injury on top of ischemic injury could cause this rapid progression. The presence or absence of recanalization on angiography was not statistically significant with respect to progression to malignant brain edema. Moreover, we did not analyze results on diffusion and perfusion MRI because 12 of our patients did not undergo MRI before IAT, so we could not assess results based on possible overlapping injuries. It is thought that more rapid progression to malignant brain edema might be the result of a complex process. Still, this study did show that the presence of a larger area of contrast enhancement on NECT performed immediately after IAT may be a crucial risk factor for such progression. Thus, clinicians would be wise to investigate the neurologic status of patients in whom a large area of enhancement may lead to the rapid development of brain edema.
Nakano et al.17) described low-density lesions detected on NECT scans obtained before IAT that correlated significantly with postoperative outcomes. However, in our study, such lesions were not analyzed because they were infrequently seen or were not clearly seen. In addition, low-density lesions on NECT after IAT were not analyzed because they were too close to areas of contrast enhancement.
The major limitations of this study are its retrospective design, single-center setting, and relatively small number of patients, which could have led to selection bias. Also, not enough imaging studies were carried out prior to IAT, so we could not adequately compare pre- and post-intervention data. However, as much as possible we carried out similar follow-up imaging studies. In addition, the procedure used for IAT varied and included different types of endovascular devices. Finally, we were unable to control the volume of contrast media injected, which is reportedly a risk factor of hemorrhagic transformation after IAT.
The extent of contrast enhancement on NECT scans obtained after IAT could be a factor in predicting progression to malignant brain edema, and patients found to have a larger area of enhancement are prone to more rapid progression. Furthermore, patients who showed large CEAR on NECT scans had a poor functional outcome at 3 months after IAT. NECT performed immediately after IAT could be helpful in allowing the clinician to assess the extent of contrast enhancement and thus prepare for possible progression to malignant brain edema.
References
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20. PMID: 25517348.
2. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke : real-world experience and a call for action. Stroke. 2010; 41:2254–2258. PMID: 20829513.

3. Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013; 368:893–903. PMID: 23390923.

4. Choi JY, Lee JI, Lee TH, Sung SM, Cho HJ, Ko JK. Emergent recanalization with stenting for acute stroke due to athero-thrombotic occlusion of the cervical internal carotid artery : a single center experience. J Korean Neurosurg Soc. 2014; 55:313–320. PMID: 25237426.

5. Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368:904–913. PMID: 23387822.

6. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke : a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384:1929–1935. PMID: 25106063.

7. Fugate JE, Klunder AM, Kallmes DF. What is meant by "TICI"? AJNR Am J Neuroradiol. 2013; 34:1792–1797. PMID: 23578670.

8. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030. PMID: 25671798.

9. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003; 34:e109–e137. PMID: 12869717.

10. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke : a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:870–947. PMID: 23370205.

11. Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009; 73:1066–1072. PMID: 19786699.

12. Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013; 368:914–923. PMID: 23394476.

13. Kim H, Jin ST, Kim YW, Kim SR, Park IS, Jo KW. Predictors of malignant brain edema in middle cerebral artery infarction observed on CT angiography. J Clin Neurosci. 2015; 22:554–560. PMID: 25510537.

14. Kim JM, Park KY, Lee WJ, Byun JS, Kim JK, Park MS, et al. The cortical contrast accumulation from brain computed tomography after endovascular treatment predicts symptomatic hemorrhage. Eur J Neurol. 2015; 22:1453–1458. PMID: 26130213.

15. Kim JT, Heo SH, Cho BH, Choi SM, Lee SH, Park MS, et al. Hyperdensity on non-contrast CT immediately after intra-arterial revascularization. J Neurol. 2012; 259:936–943. PMID: 22015965.

16. Lummel N, Schulte-Altedorneburg G, Bernau C, Pfefferkorn T, Patzig M, Janssen H, et al. Hyperattenuated intracerebral lesions after mechanical recanalization in acute stroke. AJNR Am J Neuroradiol. 2014; 35:345–351. PMID: 23907245.

17. Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion : incidence and clinical significance. Stroke. 2001; 32:2042–2048. PMID: 11546895.

18. Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia : pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007; 49:93–102. PMID: 17177065.

19. Parrilla G, García-Villalba B, Espinosa de Rueda M, Zamarro J, Carrión E, Hernández-Fernández F, et al. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke : imaging findings and clinical significance. AJNR Am J Neuroradiol. 2012; 33:1791–1796. PMID: 22538076.

20. Wildenhain SL, Jungreis CA, Barr J, Mathis J, Wechsler L, Horton JA. CT after intracranial intraarterial thrombolysis for acute stroke. AJNR Am J Neuroradiol. 1994; 15:487–492. PMID: 8197945.
21. Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004; 35:876–881. PMID: 14988575.

Fig. 1
Initial scan using non-enhanced computed tomography (NECT) shows no high-density or low-density areas in brain parenchyma (A). Angiographic results prior to intra-arterial thrombectomy (IAT) show middle cerebral artery occlusion (B). Angiographic image after IAT shows recanalization of the occlusion site (C). Immediate NECT after IAT shows contrast enhancement (D), and the contrast enhancement area ratio is greater than 0.2. Follow-up NECT after 6 hours shows malignant brain edema (E).
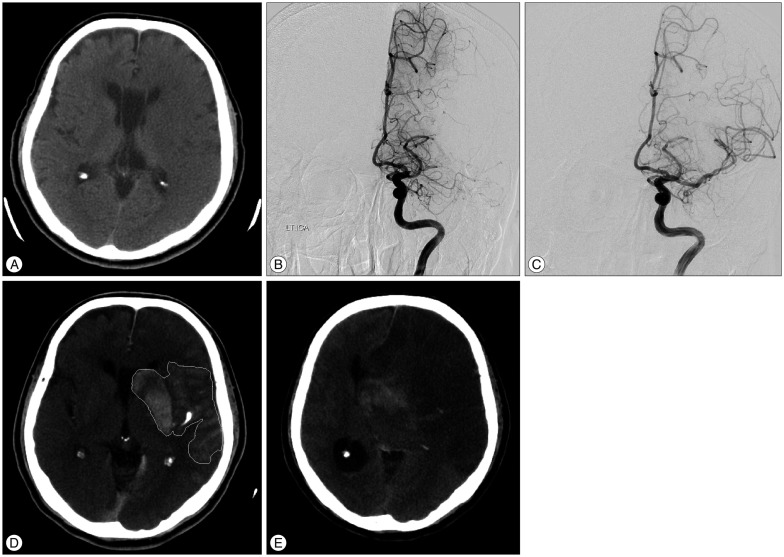
Fig. 2
Time to presence of malignant brain edema in patients with contrast enhancement area ratio (CEAR) greater than 0.2 versus those with CEAR less than 0.2.

Fig. 3
Mean modified Rankin scores in patients with CEAR ≥0.2 and those with CEAR <0.2 before acute infarction and at 3 months after IAT.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download