Abstract
Objective
This study was designed to evaluation the diagnostic value of procalcitonin (PCT) in patients with spinal infection, compare to the classical biomarkers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell (WBC) count.
Methods
All patients who were diagnosed as a spinal infection between January, 2013 and July, 2014 were included in this study. Serum PCT, CRP, ESR, and WBC count were checked at initial hospital visit and once a week serially until they were discharged. Patient's medical history, causes and pathogens of spinal infection were reviewed.
Results
Total 34 (16 men, 18 women) patients were included in this study. Mean age of the patients was 65.6 year-old. Causes of spinal infection were pain block procedure (14, 41.2%) and post-operation (5, 14.7%). Out of 25 patients who showed elevated initial serum PCT level, 20 patients (80%) had a combined systemic infection. 14 patients (6.7%) had a sepsis, 3 patients (14.2%) had a urinary tract infection and 2 (9.6%) had a pneumonia. 14 patients (41.2%) showed elevation of serum PCT level during treatment. Among them, 9 patients (64.3%) had a combined infection such as sepsis and urinary tract infection.
Traditionally spinal infection can be categorized based on anatomy : osteomyelitis, discitis, and epidural abscess. Most spinal infections spread to involve more than one of these anatomic structures4). Etiologically, spinal infection can be subdivided by spontaneous pyogenic, iatrogenic and granulomatous infection caused by fungi and parasites. Its incidence has been increasing due to elongation of life extension, aging of the population and frequency of spinal surgery or pain procedure613). Increased number of patients with diabetes mellitus, renal failure on hemodialysis and immunocompromise also affected the occurrence of spinal infection5). Diagnosis of spinal infection is based on physical examination, laboratory and radiological study. Laboratory markers, such as leukocyte counts, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), are well known inflammatory biomarkers. Recently procalcitonin (PCT) has been used to distinguish bacterial infection from non-bacterial infection10). As most of spinal infection is pyogenic, the PCT could be a help of early detecting of spinal infection. The aim of this study is to evaluate the diagnostic value of PCT in patients with spinal infection, compare to the classical biomarkers such as CRP, ESR, white blood cell (WBC) count.
Patients who were diagnosed as a spinal infection from January 2013 to July 2014 in single tertiary institute were enrolled in this study. Patients with tuberculous spondylitis were excluded for this study. The diagnosis was made with physical exam, laboratory tests and radiologic studies. Laboratory tests include infection biomarkers such as CRP, PCT, ESR, and WBC counts. CRP was measured using an immunoturbidimetric method. PCT study was based on an enzyme-linked fluorescent immunoassay. These biomarkers were examined on the admission day and at least once a week serially till they discharged.
Simple radiograph, computed tomography and magnetic resonance image (MRI) with contrast of conjectured site were checked for the radiologic studies. Additional image study was done in case of aggravating of neurologic symptoms or infection signs. Medical history of diabetes, renal disease or long term steroid use was checked. Microbiologic cultures in blood, urine and sputum were done in all patients. Culture study on patient's pus or abscess was done, if it was possible. Polymicrobial culture was considered as a contamination and excluded from the study. In case of blood culture, only single microbial culture in two sets was considered as a positive result and included in this study. Thorough systemic evaluation was performed to evaluate the co-existence of another infectious disease. Also in case of acute elevation of biomarkers during treatment, thorough evaluation was done to distinguish whether de novo infection was developed or aggravation of pre-existence spinal infection.
Total 34 consecutive patients (16 men, 18 women) were admitted our hospital during mentioned period. Their mean age was 65.6 years old (39-80 year-old). 15 patients (44.1%) had medical history of diabetes mellitus, 6 patients (17.6%) had acute or chronic renal disease, and 5 patients (14.7%) had long-term steroid therapy. Lumbar spine was the most frequently infected site (28 patients, 82.3%). Cervical (4 patients, 11.8%) and thoracic (2 patients, 5.9%) spine were followed. 12 patients (35.3%) were treated with intravenous antibiotics only and 22 patients (64.7%) had surgical removal of abscess combined with intravenous antibiotics (Table 1). Patients who showed the elevation of serum CRP or PCT level, who presented increase of abscess with neurologic symptoms in spite of intravenous antibiotics therapy had operative treatment. Patients with intractable pain even after proper conservative cares also received the operation.
Statistical analysis was performed using the SPSS 12.0 statistical software package. The quantitative variables are expressed as the median and interquartile range. The Kruskal-Wallis test was used to investigate the differences of biomarkers between groups (patients without combined infection and localized- or systemic combined infection). Differences were considered significant when the p-value was below 0.05.
The most common cause of spinal infection was spinal pain procedures (14 patients, 41.2%). Sepsis (7 patients, 20.6%), post-operative infection (5 patients, 14.7%), and urinary tract infection (5 patients, 14.7%) were followed. Cause of infection was unidentified in 3 cases (8.8%) (Table 2). Pathogen was confirmed in 22 patients (64.7%). Staphylococcus aureus (13 patients, 38.3%) was the most common pathogen of spinal infection in this study. Among this group of pathogen, 9 patients (69.2%) showed Methicillin-resistant Staphylococcus aureus (MRSA). Escherichia coli was cultured in 3 patients (8.8%) and Streptococcus viridians was cultured in 2 patients (5.9%). Candida albicans, Enterobacter, Klebsiella and Salmonella were cultured in single case (Table 3). 21 patients (61.8%) had combined infectious disease at admission. Sepsis was the most common combined infection (14 patients, 66.7%) followed by urinary tract infection (3 patients, 14.2%) and pneumonia (2 patients, 9.5%). Pharyngitis and meningitis were found in single case (Table 4).
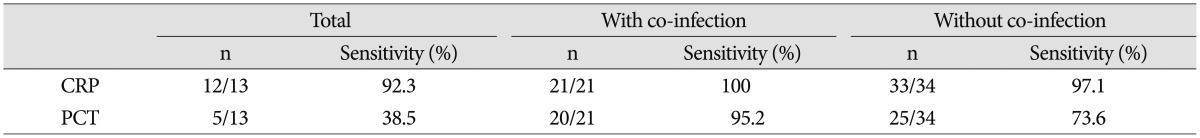
Initial results of median CRP was 12.02 mg/dL (0.31-39.28 mg/dL), median ESR was 70.5 mm/hr (33-120 mm/hr), median WBC count was 11.87 103/mL (5.08-28.44 103/mL) and median PCT was 0.195 ng/mL (0-179 ng/mL). Serum CRP level was elevated more than reference value (0-0.5 mg/dL) in 33 patients while serum PCT (reference value : 0-0.05 ng/mL) level was elevated in 25 patients. The sensitivity of CRP was 97.1% while the sensitivity of PCT was 73.6%. In patients with combined infection, however, CRP showed 100% (21/21) sensitivity and PCT showed 95.2% (20/21) sensitivity (Table 5). The reference values of WBC counts are set up as 3.5-10.0 103/mL in our hospital.
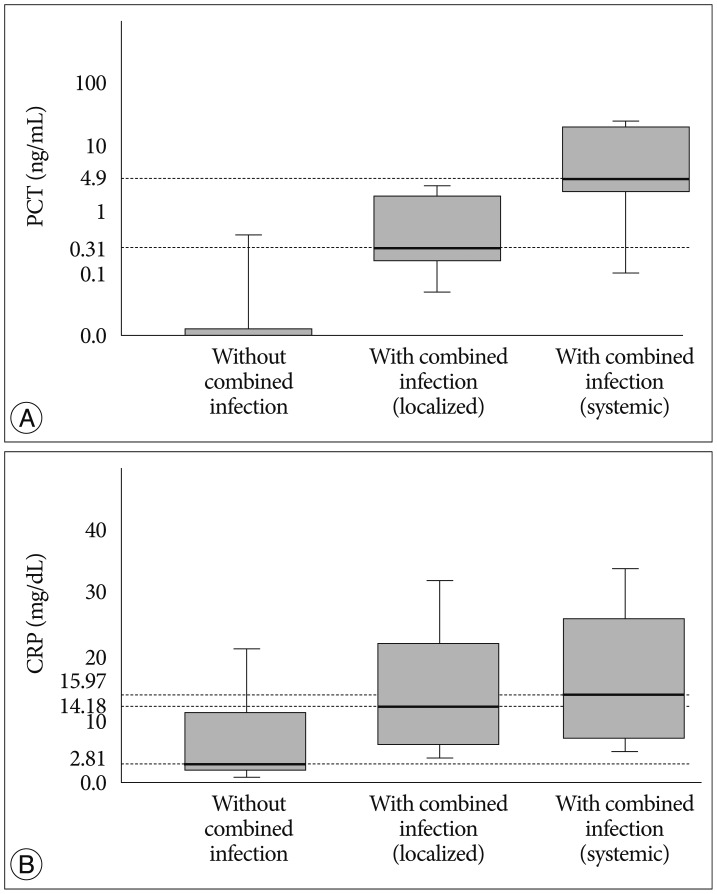
In patients group without combined infection, the median PCT was zero (0-0.73 ng/mL), the median CRP was 5.57 mg/dL (1.43-24.61 mg/dL), the median ESR was 72 mm/hr (42-120 mm/hr) and the median WBC count was 10.22 103/mL (9.45-11.59 103/mL). Among the patients with combined infection, the median PCT was 0.31 ng/mL (0-9.45 ng/mL), the median CRP was 14.18 mg/dL (2.55-33.67 mg/dL), the median ESR was 118 mm/hr (39-120 mm/hr) and the median WBC count was 12.06 103/mL (7.16-21.68 103/mL) in patients with localized infection group (clinical evidence of local infection such as positive culture of sputum, urine or the presence of clinical focus). However, in patients with systemic infection group (at least one blood culture was positive for pathogenic bacteria), the median PCT was 4.9 ng/mL (0-179.34 ng/mL), the median CRP was 15.97 mg/dL (0.31-39.28 mg/dL), the median ESR was 69 mm/hr (46-103 mm/hr) and the median WBC count was 14.19 103/mL (5.26-28.44 103/mL). Only the data of PCT in each groups was considered significant as its p-value was below 0.05 (p=0.01). Serum PCT hardly increased in cases of spinal infection without combined infection (Fig. 1).
In 14 patients (44%), serum PCT level was increased during treatment. Thorough evaluation of the reason was done. And the newly developed of sepsis (6 patients, 42.9%) was the most common reason. Occurrence of urinary tract infection (2 patients, 14.3%), drug hypersensitivity (2 patients, 14.3%) and aggravation of abscess (2 patients, 14.3%) was followed. Pharyngitis and unknown cause were the reasons in single patient. Serum PCT and CRP was gradually decreased as treatment went on. A decline was the biggest between the first and second week after treatment. PCT was decreased from 0.175 ng/mL to 0.07 ng/mL, and CRP was decreased from 10.6 mg/dL to 5.46 mg/dL. In group with surgical treatment with intravenous antibiotics, the preoperative median PCT was 0.195 ng/mL and preoperative median CRP was 10.45 mg/dL. The postoperative median PCT and CRP were decreased to 0.055 ng/mL and 6.34 mg/dL. The declination of serum PCT and CRP were shaper in patients group who underwent surgical treatment. On average, it took 83 days for normalization of CRP while 31.6 days for PCT.
Spinal infection is caused by a specific organism that involves a vertebral body, intervertebral disc or adjacent paravertebral area6). It is not a common disease and can cause serious clinical result that may lead to neurologic damage or systemic complications. The incidence is increasing in spite of development of new antibiotics and surgical technique making operation shorter. Larger proportion of the older patients with chronic disease, increase of intravenous drug abuser, and growth of spinal surgery and pain procedure could be a reason4561118).
In the past, tuberculous spondylitis is thought to be more frequent than pyogenic spondylitis5). Lee et al.11) represented 85% of spinal infection were tuberculous spinal infection in 1996. Another study done in 1999 reported that 71% of spondylitis were tuberculous12). But several recent studies report that pyogenic spondylitis is more frequent than tuberculous spondylitis58). Jeong et al.5) reported that Staphylococcus aureus was the most common pathogen in patients with pyogenic spinal infection. In our study, Staphylococcus aureus was the most frequently confirmed pathogen and especially MRSA took large part. MRSA is the pathogen that usually presents in the healthcare center. This result supports that the increase of spinal surgery and procedure leads to the raised number of infection by direct inoculation.
The early detection of infection is important in both physiologic and clinical aspects. The earlier a damaged tissue can be confirmed, the earlier preventive and therapeutic treatment can be initiated15). CRP, ESR, and WBC counts are the laboratory parameters of infectious diseases in making diagnosis and assuring the treatment response. CRP is an acute phase protein synthesized in the hepatocytes. Reference concentration of CRP was lower than 0.5 mg/dL and higher levels could be found in late pregnancy, mild inflammation, and viral infection (1-4 mg/dL). Its level could increase in active inflammation and bacterial infection (4-20 mg/dL)12). In severe bacterial infection or burn, its concentration could be over than 20 mg/dL210).
The ESR is a common laboratory test, non-specific measure of inflammation. It can be increased by any cause or focus. It increases in pregnancy or rheumatoid arthritis, and decreases in polycythemia, sickle cell anemia and congestive heart failure. ESR is sensitive marker for infection but lacks specificity117).
PCT, a prohormone for calcitonin, has emerged as a effective marker for the diagnosis of bacterial infection1021). PCT secretion begins within 4 hour after attack and peaks at 8 hour while CRP secretion starts within 4-6 hour after attack, peaking only after 36 hour1621). The clinical utility of serum PCT levels continues to evolve and nowadays there are four widespread uses of PCT level. First, the current immunoassay was approved by the FDA for establishing the likelihood of mortality in critically ill septic patients14). Second, PCT level have been used to guide empirical antibacterial therapy in patients with bronchitis, pneumonia and sepsis1920). Third, PCT level, in company with other standard biomarker, can assist in determining whether the patient's empirical antibacterial therapy is effective20). Fourth application, most useful, is the use if sequential PCT levels to determine when there is no longer a need for antibacterial therapy3).
Above these studies, Chung et al.1) reported the usefulness of PCT in the diagnosing of neurosurgical patients with postoperative fever of unknown origin. Nie et al.15) reported that PCT is more reliable marker for the early prediction of postoperative infectious complication in patients with acute traumatic spinal cord injury. Many articles have published about the usefulness of PCT39101416192021), but the study about the clinical value of PCT in spinal infection patients is rare. So we set a plan to evaluate clinical value of PCT in spinal infection patients. The significance of PCT in bacterial infection is well published in many articles and the value of PCT as early predictor or diagnostic parameter for bacterial infection is considered to be superior to that of CRP9141621). So our study was focused on the clinical significance of PCT in pyogenic spinal infection patients, not tuberculous spinal infection. It suggests that PCT is not a useful biomarker for detecting pyogenic spinal infection but a good biomarker to suspect the existence of combined infection. Though the sensitivity of PCT (25/34, 73.6%) was lower than that of CRP (33/34, 97.1%) in our study, the sensitivity of PCT (20/21, 95.2%) increased almost equally to the sensitivity of CRP (21/21, 100%) in case of combined infection. In patients group with combined infection, systemic infectious disease group showed higher sensitivity of PCT (4.9 ng/mL) than the PCT (0.31 ng/mL) of localized infectious group.
PCT is useful for monitoring of bacterial infection 1021). 14 patients experienced elevation of serum PCT level during treatment and the cause of elevation was confirmed in 13 patients. Sepsis was the most common cause and urinary tract infection was followed. Aggravtion of abscess and development of drug eruption were found in 2 cases. So in cases of elevating serum PCT during treatment of spinal infection, physicians need to evaluate the another infection focus along with spinal evaluation. We could not find other infection source that can cause elevation of PCT in one patient. Serum PCT level could increase in condition such as massive stress like severe trauma, surgery, cardiac arrest and some autoimmune disease10). These conditions could be a reason of unknown PCT elevation. Compared with CRP, the PCT concentrations were significantly different among non-combined infection, with localized combined infection and systemic infection.
Pathogens can involve spine via three routes; hemotogenous spread, direct external inoculation or spread from contiguous organ. Direct inoculation is iatrogenic caused by spinal operation, lumbar puncture or epidural procedures. Kaya et al.7) reported about 31% of patients had developed the infection after spinal surgery. In our study, spinal pain procedures (14 patients, 41.2%) were the most common cause of spinal infection. Recent tendency of increasing spinal procedures might be a reason of this result. As iatrogenic occurrence increases, Staphylococcus aureus and MRSA were the most common pathogens. Back pain is the most common complaint of the patients with spinal infection and lumbar spine is the most frequently infected site57). The degree of infection was various from focal inflammatory change to diffuse abscess formation. Sequential PCT level helps to determine when there is no longer a need for antibacterial therapy3). In our study, however, serum PCT took mead 31.6 days for nor malization while mean hospitalized period was 66.5 days. We could not stop antibiotics when PCT normalized because other clinical signs of infection were still presented.
The sensitivity of PCT was lower than that of CRP in patients with spinal infection. However, with combined infection, its sensitivity increases. Patients with systemic combined infection presented higher level of serum PCT, CRP, and WBC than patients with localized combined infection. Patients with spinal infection who showed elevated serum PCT level should be investigated for combined infection and choose the antibiotics that are targeted for spinal and other combined infection.
References
1. Chung YG, Won YS, Kwon YJ, Shin HC, Choi CS, Yeom JS. Comparison of serum CRP and procalcitonin in patients after spine surgery. J Korean Neurosurg Soc. 2011; 49:43–48. PMID: 21494362.

3. Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010; 48:2325–2329. PMID: 20421436.

4. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000; 25:1668–1679. PMID: 10870142.

5. Jeong SJ, Choi SW, Youm JY, Kim HW, Ha HG, Yi JS. Microbiology and epidemiology of infectious spinal disease. J Korean Neurosurg Soc. 2014; 56:21–27. PMID: 25289121.

6. Jung SW, Park KH, Kim TW, Kim JO, Kim JC. A clinical analysis of postoperative lumbar spondylodiscitis. Korean J Spine. 2005; 2:38–45.
7. Kaya S, Ercan S, Kaya S, Aktas U, Kamasak K, Ozalp H, et al. Spondylodiscitis : evaluation of patients in a tertiary hospital. J Infect Dev Ctries. 2014; 8:1272–1276. PMID: 25313603.

8. Kim CJ, Song KH, Jeon JH, Park WB, Park SW, Kim HB, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976). 2010; 35:E1096–E1100. PMID: 20838270.

9. Kim KE, Han JY. Evaluation of the clinical performance of an automated procalcitonin assay for the quantitative detection of bloodstream infection. Korean J Lab Med. 2010; 30:153–159. PMID: 20445333.

10. Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013; 28:285–291. PMID: 23682219.

11. Lee KS, Doh JW, Bae HG, Yun IG. Primary infections disorders of the spine : report of 40 cases. J Korean Neurosurg Soc. 1996; 25:1655–1660.
12. Lee KY, Sohn SK, Hwang KS. Comparison of pyogenic and tuberculous spondylitis. J Korean Soc Spine Surg. 1999; 6:443–450.
13. Luzzati R, Giacomazzi D, Danzi MC, Tacconi L, Concia E, Vento S. Diagnosis, management and outcome of clinically- suspected spinal infection. J Infect. 2009; 58:259–265. PMID: 19268368.

14. Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000; 28:977–983. PMID: 10809269.

15. Nie H, Jiang D, Ou Y, Quan Z, Hao J, Bai C, et al. Procalcitonin as an early predictor of postoperative infectious complications in patients with acute traumatic spinal cord injury. Spinal Cord. 2011; 49:715–720. PMID: 21243003.

16. Nijsten MW, Olinga P, The TH, de Vries EG, Koops HS, Groothuis GM, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000; 28:458–461. PMID: 10708183.

17. Olshaker JS, Jerrard DA. The erythrocyte sedimentation rate. J Emerg Med. 1997; 15:869–874. PMID: 9404806.

18. Pintado-García V. [Infectious spondylitis]. Enferm Infecc Microbiol Clin. 2008; 26:510–517. PMID: 19094866.
19. Schuetz P, Albrich W, Christ-Crain M, Chastre J, Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Rev Anti Infect Ther. 2010; 8:575–587. PMID: 20455686.

20. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions : past, present and future. BMC Med. 2011; 9:107. PMID: 21936959.
21. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection : a systematic review and meta-analysis. Clin Infect Dis. 2004; 39:206–217. PMID: 15307030.

Fig. 1
Serum PCT level (A) and CRP level (B) in patients without combined infection, with localized combined and with systemic combined infection. Median PCT level was 0 ng/mL in patients without combined infection and the result was increased to 0.31 ng/mL in patients with localized combined infection, to 4.9 ng/mL in patients with systemic combined infection (p=0.01). Median CRP was 5.57 mg/dL in patients without combined infection and it increased to 14.18 in patients with localized combined infection, to 15.97 in patients with systemic combined infection (p=0.055). Serum PCT hardly increased in cases of spinal infection without combined infection. PCT : procalcitonin, CRP : C-reactive protein.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download