Abstract
Objective
The purpose of this study is to determine whether the changes of contralateral sensorimotor cortical activation on functional magnetic resonance imaging (fMRI) can predict the neurological outcome among spinal cord injury (SCI) patients when the great toes are stimulated without notice.
Methods
This study enrolled a total of 49 patients with SCI and investigated each patient's preoperative fMRI, postoperative fMRI, American Spinal Injury Association (ASIA) score, and neuropathic pain occurrence. Patients were classified into 3 groups according to the change of blood oxygenation level dependent (BOLD) response on perioperative fMRI during proprioceptive stimulation with repetitive passive toe movements : 1) patients with a response of contralateral sensorimotor cortical activation in fMRI were categorized; 2) patients with a response in other regions; and 3) patients with no response. Correlation between the result of fMRI and each parameter was analyzed.
Results
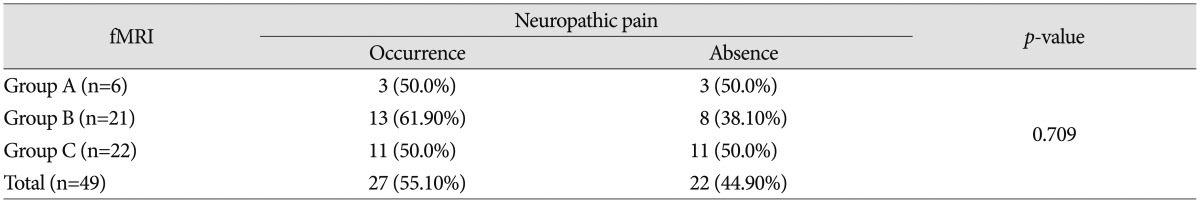
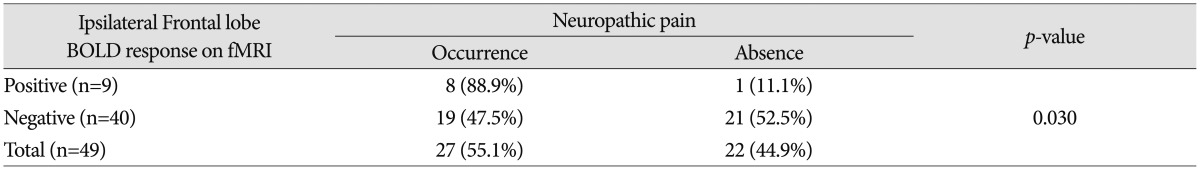
In fMRI data, ASIA score was likely to show greater improvement in patients in group A compared to those belonging to group B or C (p<0.001). No statistical significance was observed between the result of fMRI and neuropathic pain (p=0.709). However, increase in neuropathic pain in response to the signal change of the ipsilateral frontal lobe on fMRI was statistically significant (p=0.030).
Conclusion
When there was change of BOLD response at the contralateral sensorimotor cortex on perioperative fMRI after surgery, relief of neurological symptoms was highly likely for traumatic SCI patients. In addition, development of neuropathic pain was likely to occur when there was change of BOLD response at ipsilateral frontal lobe.
Patients with spinal cord injury (SCI) usually show permanent neurologic deficits and disability9). Despite development of new treatment modalities, there is still no cure for SCI12). In general, American Spinal Injury Association (ASIA) score has been used to evaluate the neurologic status and therapeutic effect of SCI patients. However, ASIA score assessment has a shortage of the prognosis in neurological outcome. Electrophysiological recordings such as somatosensory-evoked potential (SSEP) or motor evoked potential (MEP) would be helpful in early evaluation of neurological deficits and recovery of corticospinal neural integrity after SCI39). However, the use of these electrophysiological techniques for predicting neurologic outcome is still controversial8). Thus, clinical neurological examination has remained the first choice in the assessment of neurologic status and outcome89).
A spinal cord injury resulted in significant changes in the cerebral cortical activation of somatosensory and motor tasks4). Therefore, in an effort to reveal the recovery of corticospinal neural integrity after SCI, recent studies focused on functional brain mapping, such as fMRI and positron emission tomography (PET)4510). In fMRI, blood oxygenation level dependent (BOLD) response represents an upsurge, which is an indication of increased blood flow, following neural activation in the brain15). The extent of blood volume increase is topographically demonstrated in the cortex of increased brain activity15). However, the role of fMRI in evaluation of patients with SCI has not been fully evaluated4).
Neuropathic pain was recently redefined by NeuPSIG as "pain arising as a direct consequence of a lesion or disease affecting the somatosensory system"6). It is distinguished from nociceptive pain or pain caused by lesions in other parts of the nervous system (e.g., pain associated with muscular spasticity associated with lesions of central motor pathways6).
According to a recent study, the confirmatory tests of neuropathic pain are as follows : 1) Negative sensory signs (e.g., hypoesthesia and hypoalgesia) or positive sensory signs (e.g., hyperalgesia and allodynia), confined to innervation territory of the lesioned nervous structure, 2) Diagnostic test (e.g., ENMG to show the peripheral nerve lesion or MRI to show the central nervous system lesion)6). Satisfying more than two simultaneously is defined as neuropathic pain6). Neuropathic pain in SCI patients is a significant complication with a mean prevalence rate of 65%7). Despite recent advances in basic science and clinical research, the mechanisms of neuropathic pain after SCI remain unclear11).
In SCI patients, this study was conducted to determine the relationship between improvement of ASIA score and perioperative change of BOLD response on fMRI, and to analyze the correlation between perioperative change of BOLD response on fMRI and occurrence of neuropathic pain.
Seventy seven patients underwent fMRI from January 2004 to December 2013. The inclusion criteria were the following : 1) obvious SCI patients by trauma, 2) followed up more than three months, 3) underwent preoperative fMRI and postoperative fMRI, 4) no signal activation on preoperative fMRI. The exclusion criteria were the following : 1) traumatic SCI patients with accompanying brain injury, 2) patients who had cerebrovascular accident, and psychiatric disorder history, 3) patients who had neurological sequelae due to previous trauma or medical disease. A total of 49 patients were selected. Their medical records and radiological data were analyzed retrospectively. Their mean age was 43 years old (with a range of 16-69 years), and the mean follow-up period was approximately 20 months (with a range of 3-108 months). All patients underwent surgical decompression with fixation and conservative treatment. The results of preoperative and postoperative fMRI, initial and last ASIA score as well as the incidences of neuropathic pain were examined for each patient.
Changes in signal patterns in the cortical sensorimotor networks were measured using fMRI during proprioceptive stimulation with repetitive passive toe movement for all patients. The 1.5 T GE Signa Horizon scanner was utilized for fMRI. Artifacts caused by patient's movements were minimized by having a patient lie down in a supine position with eyes closed during the test. The head and limbs were restrained in order to minimize involuntary movements. Images were taken with proprio-somesthesic stimulations of the great toe. Functional images were obtained using a single-shot echoplanar imaging sequence (TR 3980 ms, interphase delay 20 ms, TE 60 ms, bandwidth 62.5 kHz, FOV 26×26 cm, matrix size 64×64, slice thickness 5 mm). There were a total of 8 periods, alternating 4 resting periods and 4 stimulating periods (proprio-somesthesic stimulation of the great toe). Following acquisition of the functional data, T1-weighted images were acquired in the same field of view. The images were analyzed by statistical parametric mapping (SPM 96, MRC Cyclotron Unit, London, UK) to correct any misalignment due to movement of patients. Gaussian filtering and high pass filtering were used to increase the signal : noise ratio and to remove noise. Functional maps were obtained through a pixel-by-pixel analysis, using a t-test with a confidence level of p<0.001, and superimposed T1-weighted images to display the activation areas. Anatomical locations of BOLD response in fMRI were classified as motor cortex, sensory cortex, thalamus, basal ganglia, temporal lobe, and the frontal lobe according to the ipsilateral or contralateral aspect of passive toe movements. The patients were categorized into 3 groups according to signal changes of perioperative fMRI (groups A, B, and C). Group A included patients who showed a BOLD response at the contralateral sensorimotor cortex area on the postoperative fMRI. Group B included patients who showed a BOLD response at the other regions on the postoperative fMRI; for example, patients who demonstrate BOLD response on fMRI either in the ipsilateral sensorimotor cortex or frontal lobe. Group C included patients who showed no BOLD response at all. There were 6 patients in group A; 21 patients in group B; and 22 patients in group C.
The occurrence of neuropathic pain was examined in all patients. In this study, neuropathic pain was defined as : 1) pain having negative sensory signs (e.g., hypoesthesia and hypoalgesia) or positive sensory signs (e.g., hyperalgesia and allodynia) arising from the sensory nerves at least 3 months after the operation, and is not a nociceptive pain, 2) obvious central nervous system injury on preoperative spine MRI.
The statistical significance of fMRI results and ASIA score improvement was examined, and statistical significance of fMRI results and neuropathic pain was tested. The chi square test and Fisher's exact test were used for evaluation of statistical significance. The results were considered significant at p-values less than 0.05. SPSS predictive analytics software was used for statistical analysis.
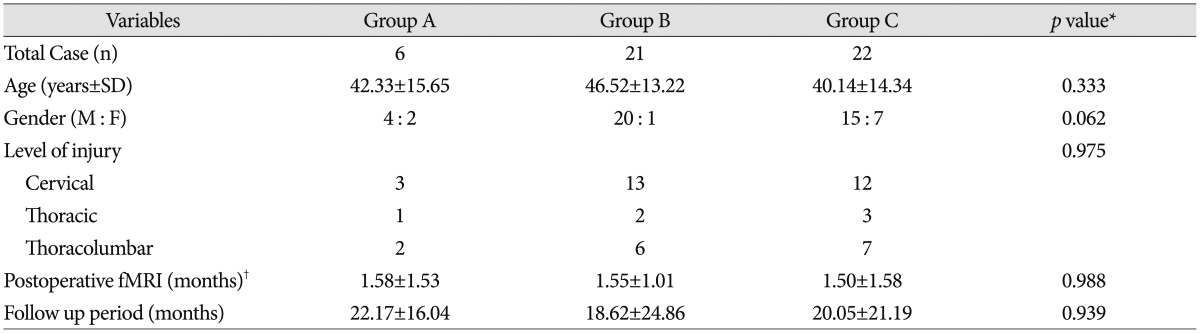
A total of 49 patients from January 2004 to December 2013 met our inclusion criteria. Among these SCI patients, 40 patients had a complete SCI (preoperative ASIA score A), while 9 patients had an incomplete SCI (preoperative ASIA score B). Forty nine patients were categorized into three groups according to change of BOLD response on perioperative fMRI. There were 6 patients in group A, with a mean age of 42.33±15.65 years (range 18-56 years) and a mean follow-up period of 22.17±16.04 months (range 4-48 months), 21 patients in group B, with a mean age of 46.52±13.22 years (range 19-69 years) and a mean follow-up period of 18.62±24.86 months (range 3-108 months), and 22 patients in group C, with a mean age of 40.14±14.34 years (range 16-66 years) and a mean follow-up period of 20.05±21.19 months (range 3-84 months). Patients' demographics and clinical data are shown in Table 1. No statistically significant difference was observed between the three groups.
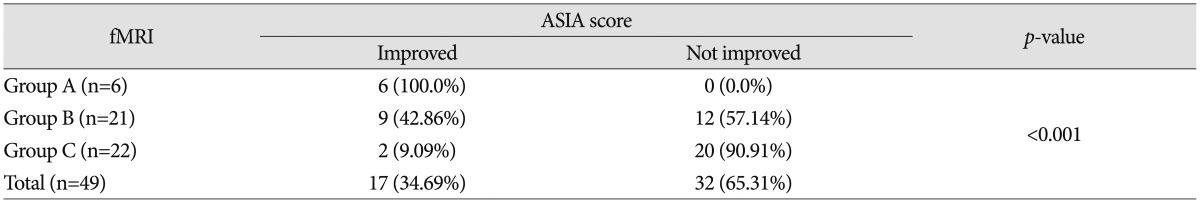
Seventeen patients (17/49; 34.69%) showed improvement of ASIA score with respect to neurologic outcome, while 32 (32/49; 65.31%) patients did not show improvement. ASIA score improvement was observed in 6 patients (100.0%) in group A, 9 patients (42.86%) in group B, and 2 patients (9.09%) in group C; 11 out of 17 patients who showed improved ASIA score were checked for ASIA score A at preoperative assessment; 6 out of 11 patients who were checked for ASIA score A at preoperative assessment improved from ASIA score A to ASIA score B. In addition, 5 out of 11 patients who were checked for ASIA score A at preoperative assessment improved from ASIA score A to ASIA score C; 6 out of 17 patients who showed improved ASIA score were checked for ASIA score B at preoperative assessment; 5 out of 6 patients who were checked for ASIA score B at preoperative assessment improved from ASIA score B to ASIA score C. The other patient improved from ASIA score B to D. According to the fMRI data, ASIA score is likely to show greater improvement when patients belong to group A than to group B or C (p<0.001) (Table 2). Statistical significance was observed in comparison of both groups A and B and groups A and C. The p-value between group A and group B was 0.020, and the p-value between group A and group C was less than 0.001, respectively. Accordingly, ASIA score improvement would depend on whether or not a patient with fMRI results belongs to group A (Fig. 1, 2).
Among 49 traumatic SCI patients, 27 patients (27/49; 55.1%) had neuropathic pain. No statistical significance in the incidences of neuropathic pain was observed between groups A, B, and C (p=0.709) (Table 3). The incidence rate of neuropathic pain tended to increase to 61.90% of cases, which belong to group B. However, no statistical significance was observed in the comparison of groups B and A and groups B and C. The p-value between groups B and A was 0.661, and the p-value between groups B and C was 0.432, respectively.
The purpose of this study is to determine whether the change of BOLD response at a specific location on perioperative fMRI affects incidence of neuropathic pain in traumatic SCI patients. The changes of BOLD response on perioperative fMRI were analyzed according to anatomical location such as motor cortex, sensory cortex, thalamus, basal ganglia, temporal lobe, and the frontal lobe. Increase in the incidence of neuropathic pain with the change of BOLD response at the ipsilateral frontal lobe on perioperative fMRI was statistically significant (p=0.030) (Table 4). In 9 patients who showed change of BOLD response at the ipsilateral frontal lobe on perioperative fMRI, development of neuropathic pain occurred in 8 patients (8/9; 88.9%), and not in the other (1/9; 11.1%) patient (Fig. 3). In contrast, among 40 patients who showed no change of BOLD response at the ipsilateral frontal lobe on perioperative fMRI, development of neuropathic pain occurred in 19 patients (19/40; 47.5%), and not in the others (21/40; 52.5%). The changes of BOLD signal at other locations of the brain were not statistically significant.
Despite significant research on the treatment of SCI patients, there is no definitive neurological examination tool for use by clinicians in assessment and prognosis of clinical outcome in SCI patients. Some studies have attempted to prove the prognostic value of electrophysiological studies, such as SSEP or MEP, however their validity and reliability are still controversial18). ASIA score has been the most widely used tool in assessment of neurologic status of patients with SCI. However, there are limitations to ASIA score for use as a prognostic factor of SCI patients. Some recent studies attempted to determine whether cortical or subcortical functional changes can occur in patients with SCI29). PET and fMRI studies using passive movement to assess the neurological status in SCI patients have been reported4910).
Dynamic neural reorganization and differentiation were reported in patients with SCI19). Several studies reported that partial recovery of motor function is related to the neural recovery of specific brain areas or pathways259). Other studies reported that the preservation of contralateral central nervous system (CNS) pathways by reorganization leads to decrease in functional deficits of SCI patients, which supports the idea of CNS plasticity14). After SCI, the change of in-and-out electrical signal patterns in the sensorimotor system causes long-term cortical or subcortical functional changes9). Increased recruitment of the contralateral or ipsilateral primary sensorimotor cortex has already been demonstrated in patients with myelitis13). In our study, there was no signal activity on preoperative fMRI of all participants during passive toe stimulation. However, after undergoing surgical decompression with fixation and conservative treatment, 27 of 49 patients showed signal changes on postoperative fMRI during passive toe stimulation. Most group A patients who showed changes of BOLD response at the contralateral sensorimotor cortex on postoperative fMRI showed neurological improvement. These findings suggest that cortical plasticity and cortical reorganization is likely to take neurological improvement in traumatic SCI patients.
Neuropathic pain is a clinical complication which commonly occurs in patients with SCI7). It is distinguished from nociceptive pain, which is caused by stimulation of peripheral nociceptive afferents like musculoskeletal pain or visceral pain. There are several hypotheses to explain the pathophysiology of neuropathic pain611). One hypothesis is that neuropathic pain is a result of abnormally suppressed inhibitory signals in the thalamus. Another hypothesis is that neuropathic pain results from maladaptive plastic changes throughout the sensorimotor neuronal pathway. Other study has reported that the neuropathic pain process would activate not only the somatosensory cortex, but also the prefrontal cortex or the anterior cingulated cortex14). Despite recent developments in basic science and clinical research, the pathophysiology of neuropathic pain after SCI is not yet clear.
Many patients with SCI suffer from neuropathic pain in both physical and psychological aspects, which may cause considerable distress such as fatigue, sleep disturbance, and mood disorders7). Neuropathic pain that occurs after spinal cord injury is less responsive to conventional medication than other types of pain11). Therefore, clinicians are required to decide in advance whether or not to control pain aggressively. In this study, no statistically significant correlation was observed between the development of neuropathic pain and the results of fMRI (p=0.709) (Table 3). However, patients had significantly higher incidences of neuropathic pain when their fMRI showed BOLD response of the ipsilateral frontal lobe (p=0.030) (Table 4). These results suggest that the results of fMRI may be helpful in predicting neuropathic pain. In SCI patients, if the occurrence of neuropathic pain can be predicted using perioperative fMRI, it may be helpful to starting the active treatment earlier.
This study had a few limitations. First, this study was designed as a retrospective review of medical records and radiological data, thus it is difficult to draw an exact conclusion between the clinical outcome and fMRI results. Second, there was no control group for evaluation of proprioception. On fMRI through passive movement of the control group (healthy subjects) in other research, the activation around the sensorimotor cortex is consistently processed4). Third, the fMRI procedure for all patients was performed by more than one person because the data were collected for 10 years. Therefore, a well designed study could provide evidence of these results. Currently research on the correlation of fMRI and neurologic outcome is limited. This article is intended to be helpful in determining the effects of fMRI results.
In patients of contralateral sensorimotor cortical activation on postoperative fMRI, the ASIA score is highly likely to show improvement. Neuropathic pain may be higher when there is a BOLD response of the ipsilateral frontal lobe on postoperative fMRI. In conclusion, this study demonstrates that perioperative fMRI change leads to expected clinical outcomes in traumatic SCI patients. Further study is necessary for evaluating the correlation between fMRI and clinical outcomes of SCI patients.
References
1. Bazley FA, Hu C, Maybhate A, Pourmorteza A, Pashai N, Thakor NV, et al. Electrophysiological evaluation of sensory and motor pathways after incomplete unilateral spinal cord contusion. J Neurosurg Spine. 2012; 16:414–423. PMID: 22303873.

2. Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002; 19:43–51. PMID: 11852977.

3. Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury : significance for predicting outcome. Spinal Cord. 1999; 37:157–165. PMID: 10213324.

4. Duggal N, Rabin D, Bartha R, Barry RL, Gati JS, Kowalczyk I, et al. Brain reorganization in patients with spinal cord compression evaluated using fMRI. Neurology. 2010; 74:1048–1054. PMID: 20200344.

5. Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury : a prospective longitudinal study. Lancet Neurol. 2013; 12:873–881. PMID: 23827394.

6. Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011; 152:14–27. PMID: 20851519.

7. Henwood P, Ellis JA. Chronic neuropathic pain in spinal cord injury : the patient's perspective. Pain Res Manag. 2004; 9:39–45. PMID: 15007402.

8. Jacobs SR, Yeaney NK, Herbison GJ, Ditunno JF Jr. Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch Phys Med Rehabil. 1995; 76:635–641. PMID: 7605182.

9. Jung JK, Oh CH, Yoon SH, Ha Y, Park S, Choi B. Outcome evaluation with signal activation of functional MRI in spinal cord injury. J Korean Neurosurg Soc. 2011; 50:209–215. PMID: 22102951.

10. Lotze M, Laubis-Herrmann U, Topka H, Erb M, Grodd W. Reorganization in the primary motor cortex after spinal cord injury - a functional Magnetic Resonance (fMRI) study. Restor Neurol Neurosci. 1999; 14:183–187.
11. Masri R, Keller A. Chronic pain following spinal cord injury. Adv Exp Med Biol. 2012; 760:74–88. PMID: 23281514.

12. McDonald JW, Becker D, Holekamp TF, Howard M, Liu S, Lu A, et al. Repair of the injured spinal cord and the potential of embryonic stem cell transplantation. J Neurotrauma. 2004; 21:383–393. PMID: 15115588.

13. Rocca MA, Agosta F, Martinelli V, Falini A, Comi G, Filippi M. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. Neuroimage. 2006; 30:879–884. PMID: 16307896.

14. Weaver KE, Richardson AG. Medial prefrontal cortex, secondary hyperalgesia, and the default mode network. J Neurosci. 2009; 29:11424–11425. PMID: 19759291.

15. Weiskopf N. Real-time fMRI and its application to neurofeedback. Neuroimage. 2012; 62:682–692. PMID: 22019880.

Fig. 1
An 18-year-old female was admitted after an automobile accident, and was confirmed to have initial ASIA score A (C4 level). A : On the initial spinal MRI, bursting fractures of C5, T3, and T4, as well as severe cord contusion were found. B : Functional MRI (fMRI) was taken 3 months after anterior and posterior fixation surgery. On the fMRI, a signal change was observed in the contralateral primary sensorimotor cortex. After surgery and 6 months conservative treatment, the ASIA score was upgraded to ASIA score B. Her follow up period was 20 months and there was no further improvement. ASIA : American Spinal Injury Association.
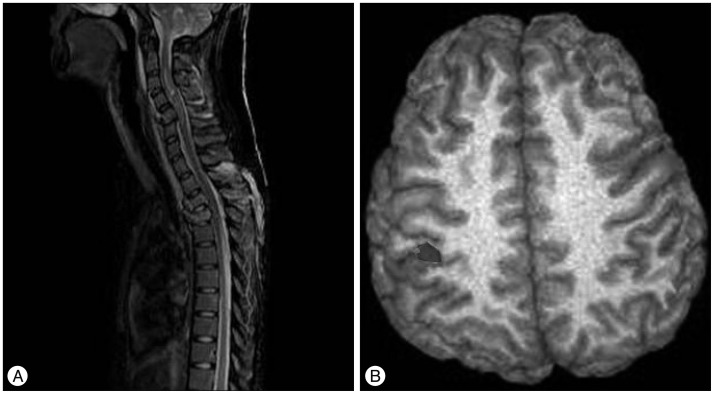
Fig. 2
A 40-year-old female was admitted to this hospital after a pedestrian traffic accident. She was confirmed to have initial ASIA score B (T8 level). A : On the initial spinal MRI, T7 bursting fracture, dislocation, and severe cord contusion were found. B : Functional MRI (fMRI) was taken 2 weeks after posterior decompression and fixation surgery. There was no positive signal in the postoperative fMRI data, despite surgery and treatment. Despite follow up of 20 months, there was no neurologic improvement. ASIA : American Spinal Injury Association.
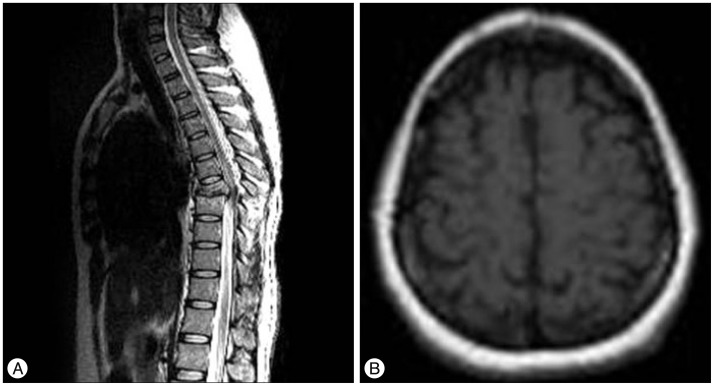
Fig. 3
A 41-year-old male was admitted after falling from a height of 5 meters. He was confirmed to have ASIA score A (T12 level). A : On the initial spinal MRI, T12 bursting fracture, dislocation, and severe cord contusion were found. B : Functional MRI (fMRI) was taken 1 week after posterior decompression and fixation surgery. In the fMRI data, there was a signal change in the ipsilateral frontal lobe. After surgery, he was followed up for 36 months, but there was no improvement of follow-up ASIA score. EMG and EP showed no abnormality, but he continued to have neuropathic pain. ASIA : American Spinal Injury Association, EMG : electromyography, EP : electrophysiology study.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download