Abstract
Objective
To investigate the efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases (BMs).
Methods
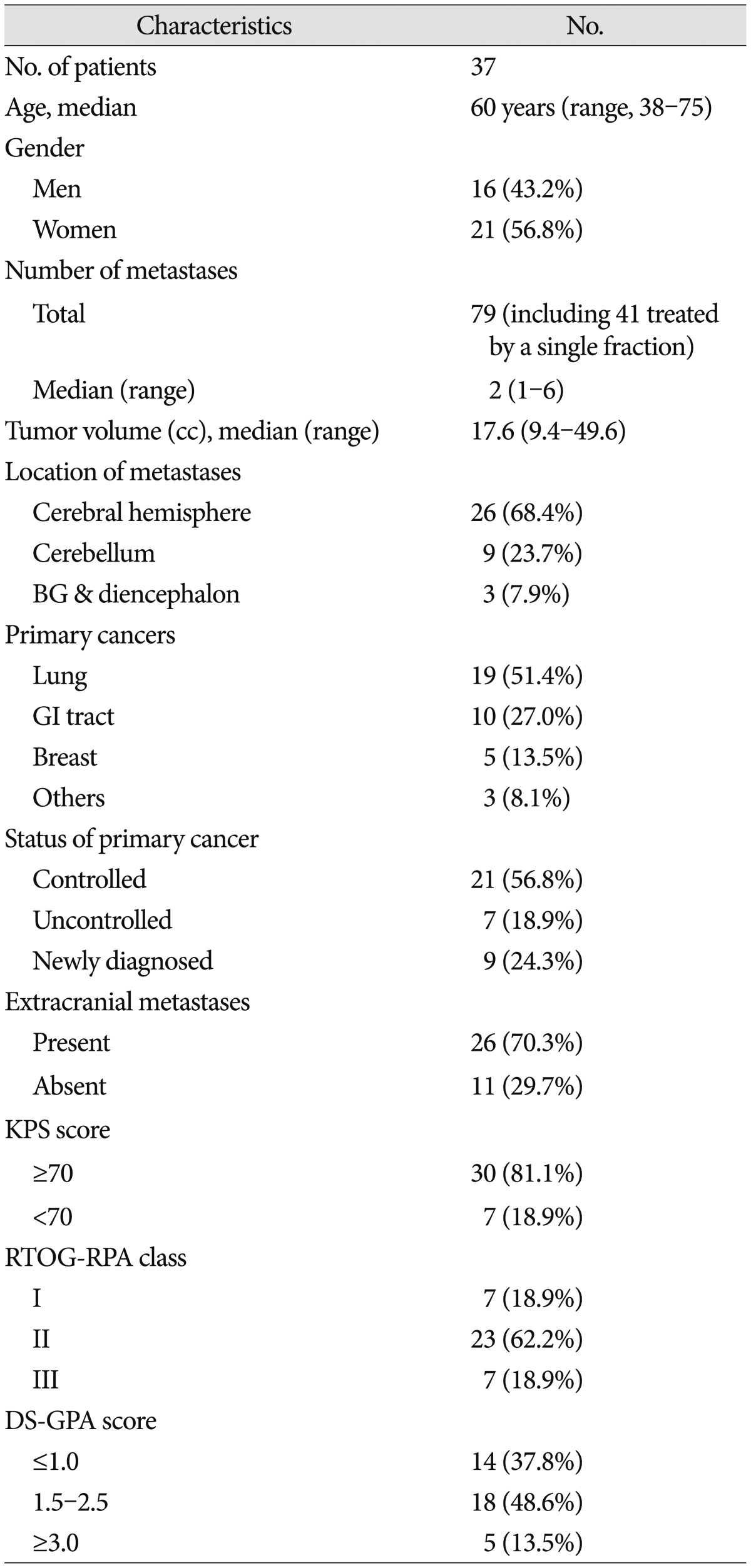
Between June 2011 and December 2013, a total of 38 large BMs >3.0 cm in 37 patients were treated with fractionated Cyberknife radiosurgery. These patients comprised 16 men (43.2%) and 21 women, with a median age of 60 years (range, 38-75 years). BMs originated from the lung (n=19, 51.4%), the gastrointestinal tract (n=10, 27.0%), the breast (n=5, 13.5%), and other tissues (n=3, 8.1%). The median tumor volume was 17.6 cc (range, 9.4-49.6 cc). For Cyberknife treatment, a median peripheral dose of 35 Gy (range, 30-41 Gy) was delivered in 3 to 5 fractions.
Results
With a median follow-up of 10 months (range, 1-37 months), the crude local tumor control (LTC) rate was 86.8% and the estimated LTC rates at 12 and 24 months were 87.0% and 65.2%, respectively. The median overall survival (OS) and progression-free survival (PFS) rates were 16 and 11 months, respectively. The estimated OS and PFS rates at 6, 12, and 18 months were 81.1% and 65.5%, 56.8% and 44.9%, and 40.7% and 25.7%, respectively. Patient performance status and preoperative focal neurologic deficits improved in 20 of 35 (57.1%) and 12 of 17 patients (70.6%), respectively. Radiation necrosis with a toxicity grade of 2 or 3 occurred in 6 lesions (15.8%).
Brain metastases (BMs) have been reported in up to 40% of patients with systemic cancer21), and the incidence of BMs is increasing due to the routine brain magnetic resonance imaging (MRI) screening and an improved outcome of systemic therapy against primary cancers. Management of BMs depends on their size, number, and location together with patient factors such as age, performance status, and primary disease status12).
Stereotactic radiosurgery (SRS), typically delivered in a single fraction, has been shown to be effective and safe in treating BMs and, is generally indicated for a single or oligometastases <3 cm in diameter. However, the toxicity of SRS given in a single fraction is considered to outweigh the benefits of local tumor control (LTC) for large BMs >3 cm and leads to increased risks of neurological morbidity from radiation necrosis (RN)31823). Although large lesions are often amenable to microsurgical resection, surgery is infeasible in cases of critical location and/or poor patient medical status.
Recently, the concept of fractionated stereotactic radiosurgery (FSRS) has emerged, and it is reportedly an effective and safe way to treat BMs, especially large lesions. Since June 2011, we adopted this approach in treating large BMs. Treatment outcomes were evaluated by the objective tumor response on MRI, patient survival and functional improvement, and radiation necrosis. Prognostic factors were also analyzed.
This study was approved by the institutional review board of the Asan Medical Center. Between June 2011 and December 2013, a total of 37 patients with large BMs were enrolled according to the inclusion and exclusion criteria indicated below.
1) Age of ≥18 years, with histologically proven solid cancer and fewer than 6 brain metastases, one of which is >3 cm in maximum diameter
2) Intact cognitive function; able to understand and sign a written informed consent
3) Life expectancy >3 months, as indicated by the medical oncologist
4) Karnofsky performance status (KPS) score ≥70, or 50-60 with focal neurological deficits
1) Suffering from significant mass effect or raised intracranial pressure for which surgical decompression is indicated
2) Received any form of prior cranial irradiation
3) Received prior surgical resection of the targeted lesion
4) Primary hematologic malignancy, such as lymphoma or leukemia
5) Pregnant or breast-feeding patients
The baseline characteristics of the study patients are summarized in Table 1. Of the 37 patients included in the analyses, 16 were men (43.2%) and 21 were women. The median age was 60 years (range, 38-75 years). BMs originated from the lung (n=19, 51.4%), gastrointestinal tract (n=10, 27.0%), breast (n=5, 13.5%), and other tissues (n=3, 8.1%). At the time of FSRS, the primary cancer was under control in 21 patients (56.8%), and metastases to organs other than the brain were present in 26 patients (70.3%). The KPS score was ≥70 in 30 patients (81.1%), and focal neurologic deficits were present in 17 patients (45.9%). There were 7 patients (18.9%) of Radiation Therapy Oncology Group-recursive partitioning analysis (RTOG-RPA) class I, 23 (62.2%) of class II, and 7 (18.9%) of class III. The diagnosis-specific graded prognostic assessment (DS-GPA) score was ≤1 in 14 patients (37.8%), 1.5-2.5 in 18 patients (48.6%), and ≥3 in 5 patients (13.5%).
Of 79 BMs in 37 patients, 38 lesions were >3 cm in diameter and were treated with FSRS. Lesions <3 cm were treated with single-fraction SRS and were not included in our analysis. The median tumor volume was 17.6 cc (range 9.4-49.7 cc). BMs were located in the cerebral hemisphere in 26 lesions (68.4%), the cerebellum in 9 lesions (23.7%), and the basal ganglia or the diencephalon in 3 lesions (7.9%) (Table 1).
All patients were treated using the Cyberknife radiosurgery system (Accuray Inc., Sunnyvale, CA, USA). Patients were immobilized using a thermoplastic mash mask. Treatment planning CT images were acquired with a 1.25 mm slice thickness. MR images including axial/sagittal/coronal T2-weighted sequences (2 mm slices) and 3D-T1-weighted gadolinium enhanced sequences (2 mm slices) were also obtained. The MRIs were registered and manually fused with the planning CT images in the Accuray MultiPlan treatment-planning system (version 4.5) to facilitate the delineation of the gross tumor volume (GTV; equal to the planning target volume) and the critical organ structures including the brainstem, the eyes, and the optic apparatus. Additional hollow structures (shells) 3 mm and 30 mm away from the GTV were generated to optimize the conformity and dose compactness. A sequential optimization method was used along with the dose objectives to optimize minimum GTV dose and coverage. A ray-tracing algorithm was used for dose calculation. The prescription isodose percentage was applied to approximately 80% of the maximum dose with a conformity index (CI, defined as the prescribed isodose volume divided by the volume of tumor encompassed by the prescription isodose volume) <1.2 and GTV coverage >99%. The median prescription dose was 35 Gy (range, 30-41 Gy). Doses were administered in 3 to 5 daily fractions depending on the size of lesions; lesions <3.5 cm were treated in 3 fractions and lesions ≥3.5 cm in 5 fractions.
Follow-up clinical examination and MRI were performed at 3-month intervals after treatment.
Tumor size was defined as the largest cross-sectional area at follow-up MRI. Each lesion was measured to evaluate local tumor response and graded using the MacDonald criteria29). Complete response was indicated by a complete disappearance of all enhancing lesions on MRI, no corticosteroid use, and clinical stability or improvement. Partial response was indicated by >50% decrease from the baseline in perpendicular diameter product sums of all measurable enhancing lesions on MRI, elimination or reduction in corticosteroid dose, and clinical stability or improvement. Progressive disease was indicated by >25% increase in perpendicular diameter product sums of enhancing lesions on MRI, appearance of a new lesion, or clinical deterioration. Patients were considered to have stable disease if they did not meet the qualifications for complete response, partial response, or progression. LTC was defined as complete response, partial response, or stable disease. Local failure was defined as radiographic progression at the treatment site. Distant failure was defined by the development of new BMs outside the treatment site.
RN was assessed objectively using MRI or confirmed pathologically after surgical resection. The following criteria were considered for RN : 1) increased T1 contrast enhancement located in the irradiated area with central hypointensity and increased peripheral edema, 2) substantial regression or stability (for at least 3 months) of enhancing areas on serial follow-up MRIs without additional treatment, or 3) absence of perfusion within the contrast-enhancing lesion on dynamic susceptibility contrast perfusion MRI19). All radiation toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE Version 4.0).
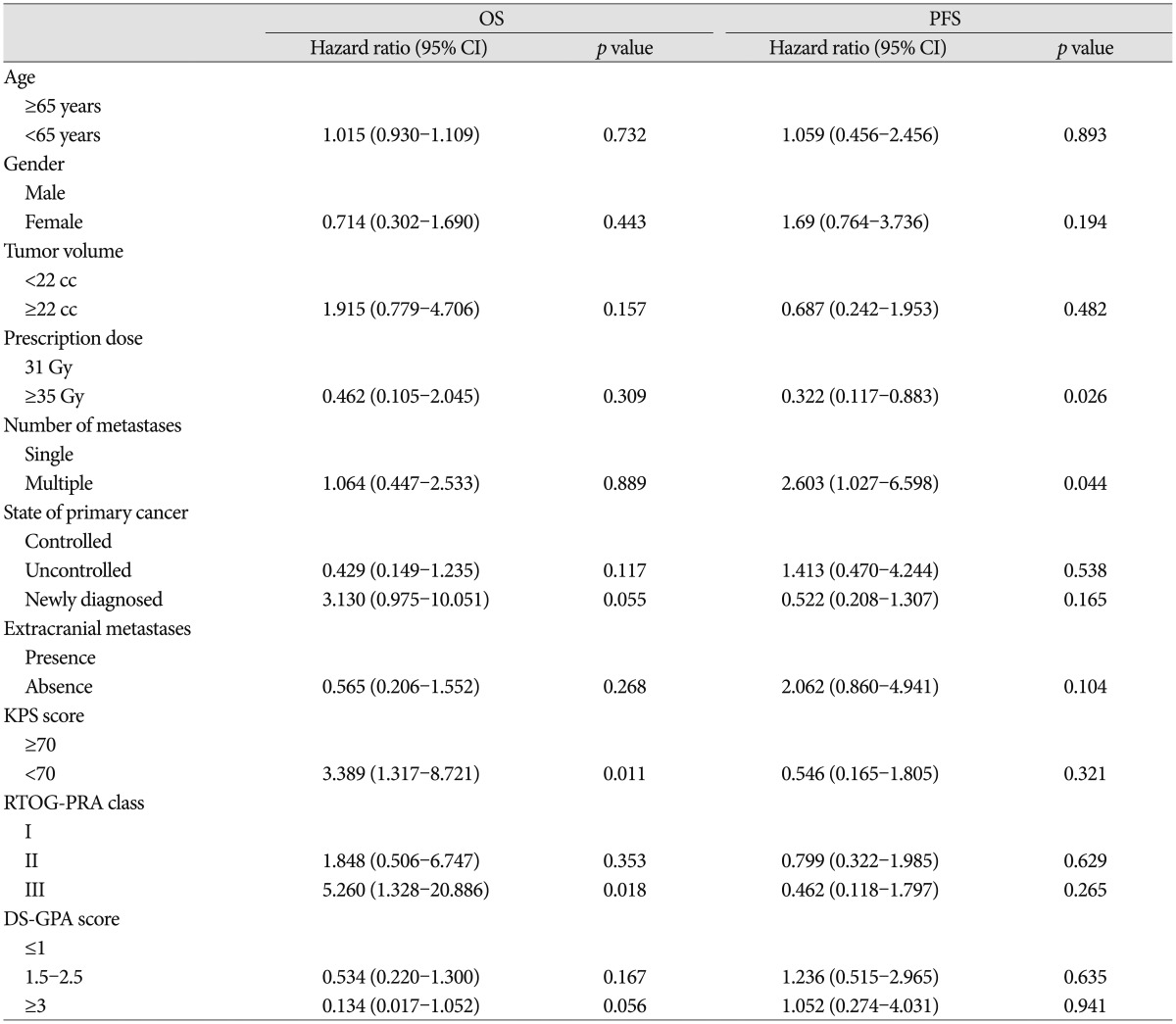
LTC, overall survival (OS), progression-free survival (PFS), and RN were estimated using the Kaplan-Meier method calculated from the treatment start date to the date of events or the last follow-up. Factors possibly affecting the outcome were tested using the log-rank test for univariate analysis and the Cox proportional hazards models with variable selection, which included age (≥65 years vs. <65 years), gender, primary cancer type, tumor location, tumor volume (<22 cc vs. ≥22 cc), single vs. multiple BMs, status of primary cancer, presence of extracranial metastases, pretreatment KPS score (≥70 vs. <70), ROTG-RPA class, DS-GPA score, and prescription dose. All statistical tests were conducted using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p<0.05.
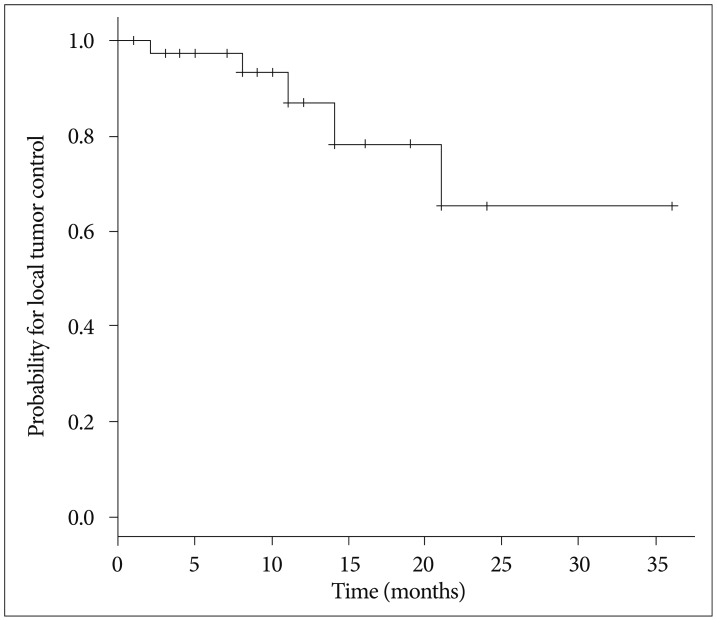
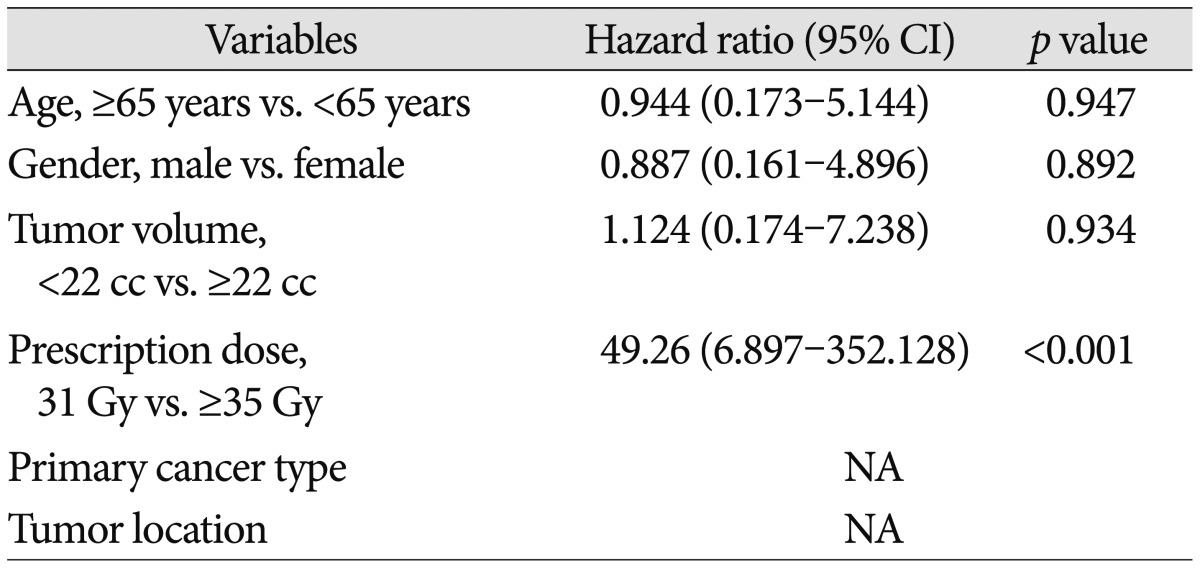
The maximum tumor response was evaluated for 36 lesions after exclusion of two lesions for which no follow-up images were available. The rates of complete response, partial response, stable disease, and progressive disease were 11.1%, 44.4%, 30.6%, and 13.9%, respectively. With a median follow-up of 10 months (range, 1-37 months), the crude LTC rate was 86.8% and the estimated LTC rates at 12 and 24 months were 87.0% and 65.2%, respectively (Fig. 1). Prescription dose was the only factor affecting the LTC on univariate and multivariate analysis. Both of the lesions treated with a prescription dose of 31 Gy developed local failure, whereas only 3 of 36 lesions with a prescription dose of ≥35 Gy developed local failure (hazard ratio, 49.26; 95% confidence interval, 6.897-352.128; p<0.001) (Table 2).
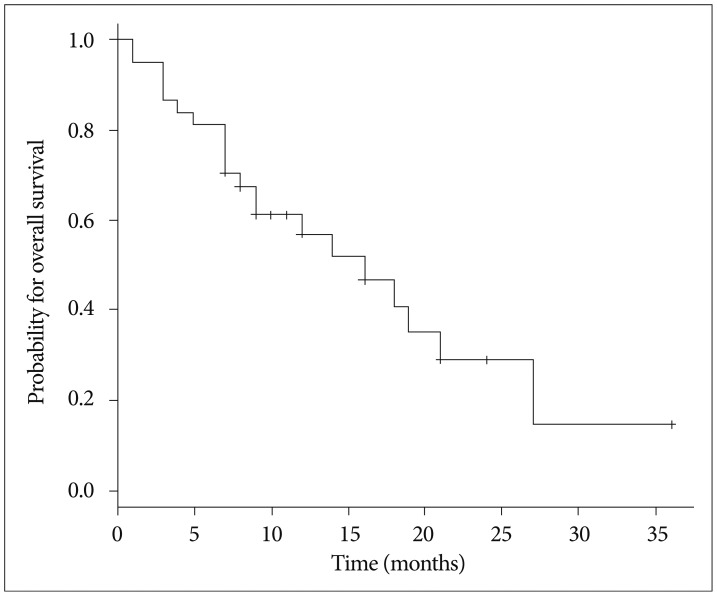
The Kaplan-Meier curve for OS is shown in Fig. 2. The median OS was 16 months, and the estimated OS rates at 6, 12 and 18 months were 81.1%, 56.8%, and 40.7%, respectively. Of 21 patients who died, 10 (47.6%) died from the progression of extracranial disease, 6 (28.6%) from brain failure, and 5 (23.8%) from unknown causes. On univariate analysis, KPS score <70 (hazard ratio, 3.389; 95% confidence interval, 1.317-8.721; p=0.011) and RTOG-RPA class III (hazard ratio, 5.26; 95% confidence interval, 1.328-20.886; p=0.018) indicated poor patient survival (Table 3, Fig. 3), although only RTOG-RPA class remained significant on multivariate analysis.
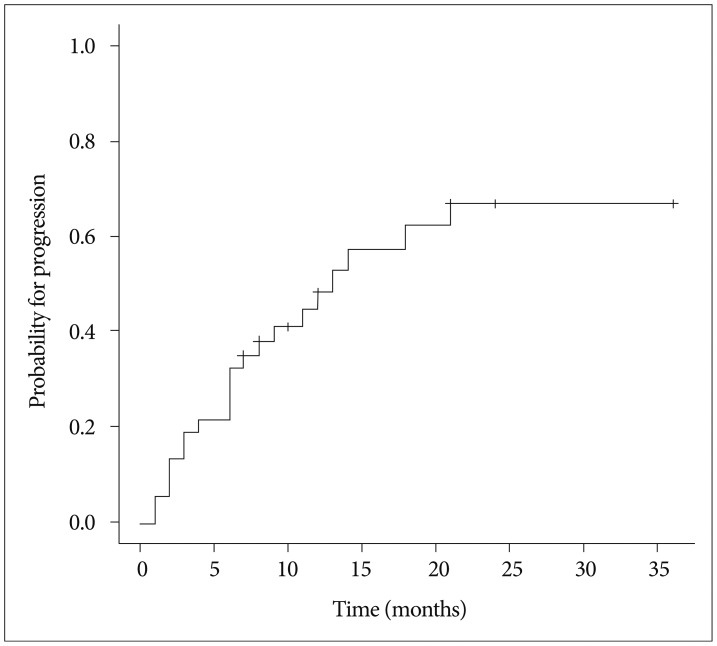
Twenty-one patients (56.8%) showed progression including distant failure in 20 patients, local failure in 5 patients, and both distant and local failure in 4 patients. The median PFS was 11 months and the estimated PFS rates at 6, 12, and 18 months were 65.5%, 44.9%, and 25.7%, respectively. The cumulative incidence function (CIF) for progression is shown in Fig. 4. Multiple BMs were associated with poor PFS (hazard ratio, 2.603; 95% confidence interval, 1.027-6.598; p=0.044) (Table 3), as 14 of 19 patients (73.7%) with multiple BMs developed progression vs. 7 of 18 (38.8%) with a single BM.
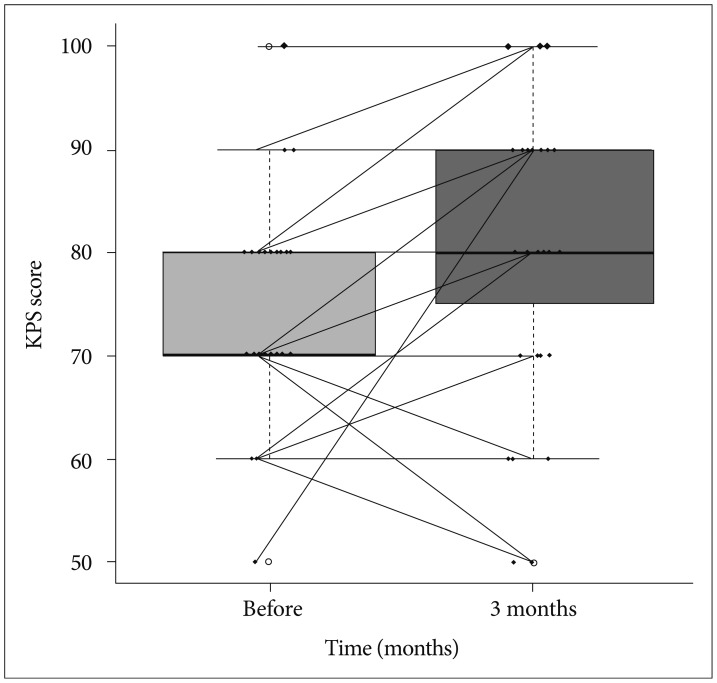
Preoperative focal neurologic deficits such as motor weakness and cerebellar dysfunction, improved in 12 of 17 patients (70.6%) 3 months after treatment. The KPS score improved in 20 of 35 patients (57.1%), with a mean preoperative KPS score of 74 (median, 70; range, 50-100) vs. a mean KPS score of 80.6 (median, 80; range, 50-100) 3 months after treatment (p=0.001) (Fig. 5).
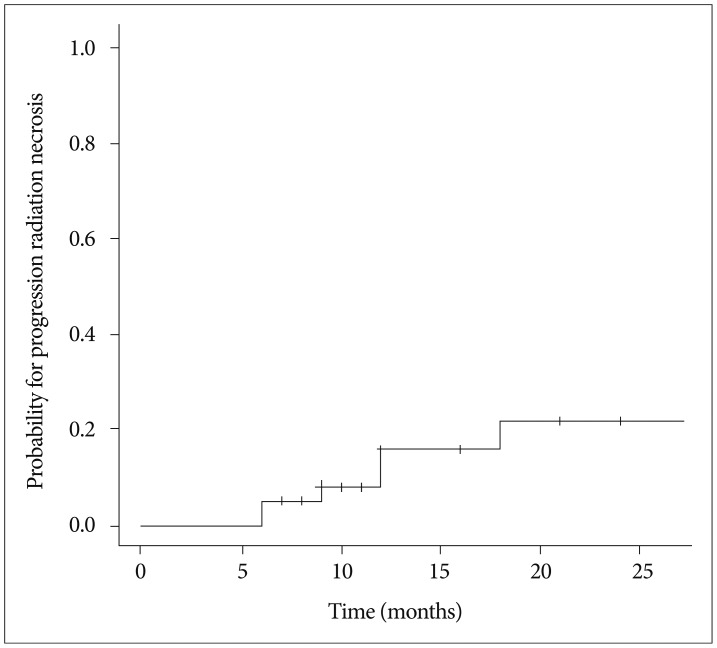
RN occurred in 6 of 38 lesions (15.8%). CIF for RN is shown in Fig. 6. The median time to RN was 10.5 months (range, 6-18 months). Five patients with RN of toxicity grade 2 were controlled with corticosteroid medication and 1 patient with toxicity grade 3 was salvaged by surgery. No factors were identified that affected the occurrence of RN.
Although SRS typically delivered in a single fraction has been proven to be effective and safe in treating BMs, it is not feasible for large lesions, especially those >3.0 cm, due to increased toxicity and local treatment failure3182324). Microsurgical resection is usually indicated for large BMs, immediately decompressing the mass effect and alleviating neurological symptoms. However, not all patients with large BMs are eligible for surgery when considering surgical accessibility, the number of lesions, and patient medical status1733). Moreover, systemic therapy against primary cancers should be withheld during perioperative periods, which can be further confounded by surgical morbidity in certain cases. Alternatively, whole-brain radiotherapy (WBRT) remains palliative in nature and may influence cognitive function. Currently, the first-line treatment for large BMs has not been established and is usually determined by considering various factors, including tumor volume, number, location, and overall condition of patient12).
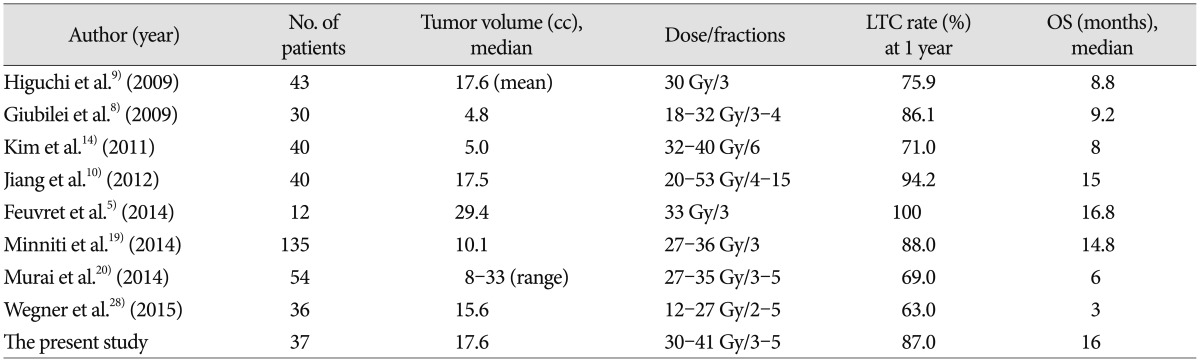
Theoretically, fractionated administration of radiation dose potentially minimizes toxicity to late-responding healthy tissues, with a low α/β ratio compared to a single acute dose of radiation for a given level of tumor damage, according to the linear quadratic model of cellular survival826). In addition, reoxygenation and redistribution of the cell cycle between dose fractions renders hypoxic tumor cells, which are abundant in large BMs compared to small tumors, more radiosensitive6142225). As expected, recently published studies on FSRS for large BMs have demonstrated high LTC rates, ranging from 63-100%, at 1 year follow-up, with acceptable risks of toxicity1410141531) (Table 4). Consistent with these results, our present LTC rates were 87.0% and 65.2% at 1- and 2-years follow-up, respectively, and the median OS was 16 months, which also compares well with the outcomes of single-fraction SRS for small BMs2111316). Furthermore, patient performance status and neurological function improved significantly, presumably benefitting the quality of life in these cases.
The optimal dose fractionation protocol for FSRS in BMs has not yet been established. In a recent systematic review on stereotactic radiotherapy dose and LTC probability, Wiggenraad et al.30) reported that a biological effective dose, using an α/β ratio of 12 (BED12), of at least 40 Gy, which correspond to a single fraction dose of 20 Gy, was associated with a 1-year LTC rate of 70% or more. The high LTC rate at 1-year follow-up in our present study appears to accord with this observation. Lower LTC rates have been also associated with large BMs2732). Vogelbaum et al.27) reported a 1-year LTC rate of 45% for lesions of 3.1-4.0 cm in diameter vs. 85% for lesions ≤2.0 cm with a prescription dose of 15 Gy. In our present analyses, which included only large BMs, the overall LTC rates observed were comparable to historic single fraction SRS for small BMs.
The LTC rates of even larger lesions in our current series (≥3.5 cm) treated with more fractions were not inferior to those of lesions <3.5 cm, indicating a promising role of FSRS in treating large BMs. Recently, Murai et al.20) reported that dose fractionation of 27-30 Gy in 3 fractions and 31-35 Gy in 5 fractions on consecutive days was tolerable and effective in treating large BMs. Further studies are needed to determine the optimal dose fractionation protocol, especially in relation to tumor size.
RN has been reported at a rate of 2-15% after FSRS1410141531). In our current study, RN occurred in 6 out of the 38 lesions we examined (15.8%), which falls at the upper margin of this range. This can be explained in part by a slightly higher prescription dose employed for our present cases and lack of uniform criteria for RN in different studies. Meanwhile, most of our patients with RN were controlled with corticosteroid medication, except for one instance salvaged by surgery. As the incidence of brain necrosis after SRS increases with the size of the target volume, the volume of normal brain receiving a certain threshold dose has been implicated in the development of RN3181923), with smaller volumes having a lower risk of RN.
In line with previous studies, our multivariate analysis showed that good patient performance (KPS score ≥70) and lower RTOG-RPA class significantly predicted a better survival outcome. Gaspar et al.7) reported that RTOG-RPA class I cases had the best survival outcomes (median 7.1 months), whereas those of ROTG-RPA class III had the poorest survival results (median 2.3 months). Kim et al.14) reported that good KPS (≥70), controlled primary cancer, no extracranial metastases, lower RTOG-RPA class, higher DS-GPA score, single brain metastasis, and absence of previous WBRT were significant predictors of longer survival, Of these variables, only the number of extracranial metastatic organs was found to be a only significant predictor in our multivariate analysis. Minniti et al.19) reported previously that stable extracranial disease and a good KPS (>70) were associated with the most significant survival benefit.
FSRS is now emerging, yet controversial and not a current standard of practice in treating large BMs. This study presents additional clinical data that support the application of this approach as valid modality in terms of efficacy and safety.
Acknowledgements
This paper was presented at the 54th annual meeting of the Korean Neurosurgical Society, held from Oct 17 to Oct 19, 2014.
References
1. Aoyama H, Shirato H, Onimaru R, Kagei K, Ikeda J, Ishii N, et al. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003; 56:793–800. PMID: 12788187.

2. Bir SC, Ambekar S, Nanda A. Long term outcome of Gamma Knife radiosurgery for metastatic brain tumors. J Clin Neurosci. 2014; 21:2122–2128. PMID: 25065951.

3. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010; 77:996–1001. PMID: 19783374.

4. Fahrig A, Ganslandt O, Lambrecht U, Grabenbauer G, Kleinert G, Sauer R, et al. Hypofractionated stereotactic radiotherapy for brain metastases--results from three different dose concepts. Strahlenther Onkol. 2007; 183:625–630. PMID: 17960338.

5. Feuvret L, Vinchon S, Martin V, Lamproglou I, Halley A, Calugaru V, et al. Stereotactic radiotherapy for large solitary brain metastases. Cancer Radiother. 2014; 18:97–106. PMID: 24439342.

6. Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994; 28:797–802. PMID: 8138431.

7. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997; 37:745–751. PMID: 9128946.

8. Giubilei C, Ingrosso G, D'Andrea M, Benassi M, Santoni R. Hypofractionated stereotactic radiotherapy in combination with whole brain radiotherapy for brain metastases. J Neurooncol. 2009; 91:207–212. PMID: 18807149.

9. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, et al. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009; 74:1543–1548. PMID: 19135317.

10. Jiang XS, Xiao JP, Zhang Y, Xu YJ, Li XP, Chen XJ, et al. Hypofractionated stereotactic radiotherapy for brain metastases larger than three centimeters. Radiat Oncol. 2012; 7:36. PMID: 22429918.

11. Jo KI, Im YH, Kong DS, Seol HJ, Nam DH, Lee JI. Gamma knife radiosurgery for brain metastases from breast cancer. J Korean Neurosurg Soc. 2013; 54:399–404. PMID: 24379946.

12. Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, et al. The role of surgical resection in the management of newly diagnosed brain metastases : a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:33–43. PMID: 19960230.

13. Kim H, Jung TY, Kim IY, Jung S, Moon KS, Park SJ. The usefulness of stereotactic radiosurgery for radioresistant brain metastases. J Korean Neurosurg Soc. 2013; 54:107–111. PMID: 24175024.

14. Kim YJ, Cho KH, Kim JY, Lim YK, Min HS, Lee SH, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 2011; 81:483–489. PMID: 20800386.

15. Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009; 115:890–898. PMID: 19132728.

16. Lee CC, Yen CP, Xu Z, Schlesinger D, Sheehan J. Large intracranial metastatic tumors treated by Gamma Knife surgery : outcomes and prognostic factors. J Neurosurg. 2014; 120:52–59. PMID: 24160478.

17. Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005; 63:37–46. PMID: 16111570.

18. Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases : analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011; 6:48. PMID: 21575163.
19. Minniti G, D'Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, et al. Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol. 2014; 117:295–301. PMID: 24488446.

20. Murai T, Ogino H, Manabe Y, Iwabuchi M, Okumura T, Matsushita Y, et al. Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases : a dose escalation study. Clin Oncol (R Coll Radiol). 2014; 26:151–158. PMID: 24332223.

21. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003; 29:533–540. PMID: 14585263.

22. Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994; 29:427–431. PMID: 8005794.

23. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases : final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000; 47:291–298. PMID: 10802351.

24. Shehata MK, Young B, Reid B, Patchell RA, St Clair W, Sims J, et al. Stereotatic radiosurgery of 468 brain metastases < or = 2 cm : implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004; 59:87–93. PMID: 15093903.

25. Shibamoto Y, Yukawa Y, Tsutsui K, Takahashi M, Abe M. Variation in the hypoxic fraction among mouse tumors of different types, sizes, and sites. Jpn J Cancer Res. 1986; 77:908–915. PMID: 3095287.
26. Tomé WA. Universal survival curve and single fraction equivalent dose : useful tools in understanding potency of ablative radiotherapy : in regard to Parks et al. (Int J Radiat Oncol Biol Phys 2008;72 : 1620-1621). Int J Radiat Oncol Biol Phys. 2008; 73:1286. PMID: 19251106.

27. Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006; 104:907–912. PMID: 16776334.

28. Wegner RE, Leeman JE, Kabolizadeh P, Rwigema JC, Mintz AH, Burton SA, et al. Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol. 2015; 38:135–139. PMID: 23563213.

29. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas : response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28:1963–1972. PMID: 20231676.

30. Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011; 98:292–297. PMID: 21316787.

31. Wiggenraad R, Verbeek-de Kanter A, Mast M, Molenaar R, Kal HB, Lycklama à Nijeholt G, et al. Local progression and pseudo progression after single fraction or fractionated stereotactic radiotherapy for large brain metastases. A single centre study. Strahlenther Onkol. 2012; 188:696–701. PMID: 22722818.

32. Yang HC, Kano H, Lunsford LD, Niranjan A, Flickinger JC, Kondziolka D. What factors predict the response of larger brain metastases to radiosurgery? Neurosurgery. 2011; 68:682–690. discussion 690PMID: 21311296.

33. Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009; 110:730–736. PMID: 19072310.

Fig. 3
Overall survival outcomes by Karnofsky performance status (KPS) score (A) and Radiation Therapy Oncology Group-Recursive Partitioning Analysis (RTOG-RPA) class (B).
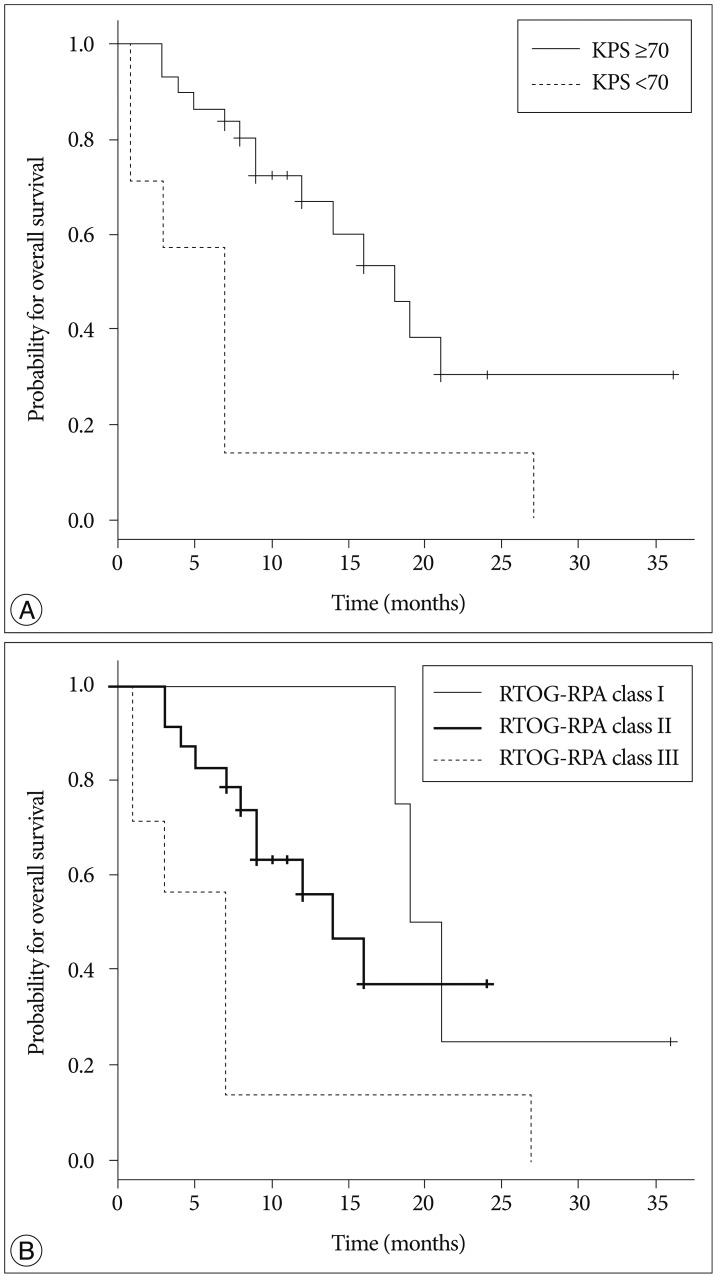
Fig. 5
Changes in the Karnofsky performance status (KPS) score 3 months after fractionated stereotactic radiosurgery. Upper and lower margins of the box indicate 75th and 25th percentile, respectively. Bold lines in the box indicate the median value.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download