Abstract
Objective
This study was aimed at optimizing the treatment of non-small-cell lung cancer (NSCLC) patients who are candidates for stereotactic radiosurgery (SRS) for brain metastases and harbor activating epithelial growth factor receptor (EGFR) mutations.
Methods
We retrospectively reviewed the medical records from 2005 to 2010 of NSCLC patients with brain metastases harboring an activating EGFR mutation. Patients who received a combination therapy of SRS and EGFR-tyrosine kinase inhibitor (TKI) for brain metastases and those who received SRS without EGFR-TKI were compared. The primary endpoint was progression-free survival (PFS) of the brain metastases.
Results
Thirty-one patients were eligible for enrolment in this study (SRS with TKI, 18; SRS without TKI, 13). Twenty-two patients (71.0%) were women and the median overall age was 56.0 years. PFS of brain lesions was not significantly prolonged in SRS with TKI treatment group than in SRS without TKI group (17.0 months vs. 9.0 months, p=0.45). Local tumor control rate was 83.3% in the combination therapy group, and 61.5% in the SRS monotherapy group (p=0.23). There were no severe adverse events related with treatment in both groups.
Lung cancer is the most frequent cause of cancer-related death worldwide23). Brain metastases occur in 20-40% of non-small-cell lung cancer (NSCLC) patients and are one of the most important malignancy-related issues in these cases because of the considerable mortality and morbidity that result1226). Although whole-brain radiotherapy (WBRT) has traditionally been used for treating brain metastases, it cannot be used in all cases because of its adverse effects and complications19). Moreover, it is well known that conventional chemotherapy is not effective for brain metastases because of the blood-brain barrier. Recently, stereotactic radiosurgery (SRS) has been introduced as an effective substitute for WBRT in selective patients who have single or multiple small-sized metastatic lesions in the brain71422). In addition, tyrosine kinase inhibitors (TKIs) have shown efficacy in patients with brain metastases from an activating epithelial growth factor receptor (EGFR)-mutant NSCLC321). The efficacy of concurrent erlotinib plus WBRT was previously investigated in both wild-type and EGFR-mutant patients, and the median survival of the patients with an EGFR mutation was much longer than those with wild-type EGFR (19.1 months vs. 9.3 months)27). Thus, the EGFR mutation status is an important determinant of the effectiveness of TKIs. However, there are no available data on the optimal brain metastases treatment for EGFR-mutant NSCLC.
This study was performed to determine if a concurrent SRS and TKI therapy is more effective than SRS alone in patients who were indicated SRS for brain metastases from an activating EGFR-mutant NSCLC.
We retrospectively identified patients with measurable brain metastases from a pathologically confirmed NSCLC harboring an activating mutation in EGFR who were treated with SRS for the brain tumor at our institution between 2005 and 2010. Patients with brain metastases derived from other cancers were excluded. SRS treatment was indicated when there were fewer than ten brain metastases, each less than 3 cm in size, on contrast-enhanced brain magnetic resonance imaging (MRI). The following cases were included in the present study : synchronous brain metastases at first diagnosis, relapse of brain metastases after primary site surgery, and new development of a brain lesion following chemotherapy. In addition, patients were required to be over 18 years of age, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-3, regardless of neurological symptoms. Patients with severe comorbid conditions and other active malignancies were excluded. Patients who had received prior WBRT or TKI treatment were excluded from the study. The study was approved by the Institutional Review Board of the Asan Medical Center.
All specimens for EGFR mutation analysis were primary lung cancer tissues. Among them, 23 (74.2%) were from biopsy tissue and others (25.8%) were the specimens from operation. DNA was derived from tumors embedded in paraffin blocks using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). Exons 18, 19, and 21 of the tyrosine kinase domain of the EGFR-coding sequence were amplified by polymerase chain reaction (PCR) with specific primers. PCR products were sequenced directly using an automatic ABI PRISM Genetic Analyzer 3130 (PE Applied Biosystems, Foster City, CA, USA).
All metastatic tumors in the brain were treated with SRS using Leksell Gamma Knife model C (Elekta, Stockholm, Sweden). We obtained T1-weighted, high-dose gadolinium enhanced images, with an injected gadolinium volume of 0.4 mmol/kg. We used multiple isocenters to insure a highly conformal dose distribution and defined a prescription dose as a dose covering at least 93% of the target volume. To irradiate the tumor margins, the median peripheral dose of 23 Gy (range, 15-25 Gy) was prescribed at the median 50% isodose line (range, 34-65%). After SRS, the patients were observed to detect any acute adverse effects.
Patients treated with SRS and TKIs for brain metastases received the TKIs within 2 weeks of commencing SRS. These patients received oral gefitinib (Iressa; AstraZeneca, London, UK) 250 mg or erlotinib (Tarceva; Roche, Basel, Switzerland) 150 mg once daily. The other patients were treated with systemic chemotherapy. The guideline recommendations about application of EGFR-TKI treatment for lung cancer patients harboring activating EGFR mutation were changed from the second line treatment to the first line treatment in Korea in 2010. Among various systemic therapy, regimen was chosen for each patient regarding patients' preference, side effect and previous treatment history.
Brain MRI was immediately performed when new neurologic symptoms or signs developed or every 3 months. Objective tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.025). Partial response (PR) was defined as at least a 30% decrease in the sum of the longest target lesion diameter, taking as reference the longest baseline diameter and/or the persistent of one or more non-target lesion. Progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameter, taking as a reference the smallest sum of the longest diameter recorded after treatment, or the appearance of one or more new lesions in brain MRI. Stable disease (SD) was defined as the absence of significant shrinkage or enlargement qualifying complete response or PR or PD.
Adverse events were followed closely thoughout 24 hours after SRS with particular interest in neurological deficits. Adverse events were the newly developed symptoms that patients complained after SRS. Severe adverse events were defined as life threatening events from any cause after procedure or other symptoms such as headache that would lead physicians either to treat brain edema with steroid or surgery.
Frequencies and descriptive statistic of demographic and clinical variables were obtained. Overall survival (OS) from the time of brain metastasis to death was estimated using the Kaplan-Meier method. Progression-free survival (PFS) was defined as time interval from the SRS treatment to documented disease progression in the central nervous system (CNS) or death from any cause. Continuous variables were compared using a Student's t-test. Statistical analyses for categorical variables were performed using a chi-square test or Fisher's exact test when the expected value was less than five. All analyses were conducted using SPSS software for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was determined by a two-tailed p-value of less than 0.05.
Of the 316 patients treated with SRS for brain metastases from primary NSCLCs, information on EGFR mutations was available for 138 cases. Of these, 50 patients (36.2%) had an activating EGFR mutation. Eleven patients were excluded because they had previously received EGFR-TKI before the brain metastasis arose, had received no systemic therapy, or had no follow-up data (Fig. 1). Eighteen patients received concurrent SRS and TKI treatment and thirteen received SRS without TKI. Baseline characteristics of our study patients are listed in Table 1. Of these 31 patients, 22 (71.0%) were women. The median age of these enrolled patients was 56.0 years. The histological subtype in each case was adenocarcinoma with an activating EGFR mutation. Deletion in exon 19 was the most common type (48.4%) of EGFR mutation in our study series, followed by the L858R point mutation (45.2%). The T790M mutation, known as the EGFR-TKI resistance mutation, was found in one patient in each group. Brain metastasis without other systemic lesions was found in three patients after surgical treatment of the lung cancer. The median number of brain metastases was 2 (range, 1-8) and the median tumor volume was 1.30 cc (range, 0.05-16.10 cc).
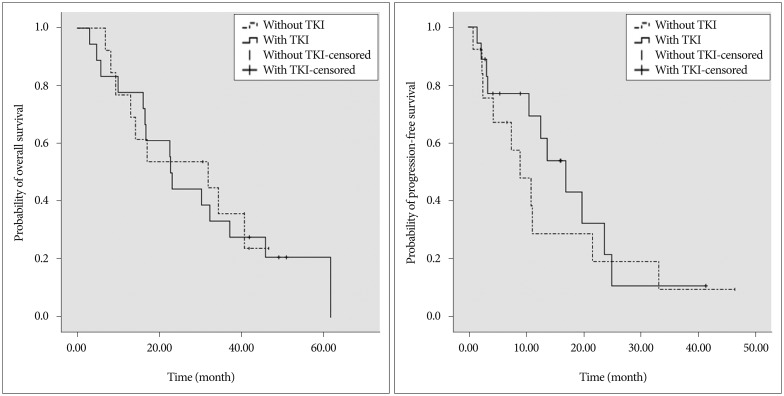
TKI was administered as a first-, second-, and third-line treatment in 5 (27.8%), 11 (61.1%), and 2 (11.1%) of the study patients, respectively. A doublet regimen of gemcitabine and cisplatin was administered in four patients (30.8%) and paclitaxel with cisplatin, vinorelbine, docetaxel, and gemcitabine monotherapy were used for each of two patients (15.4%). The median duration of TKI medication was 13.4 months. Five patients (27.8%) in the SRS with EGFR-TKI group and two (15.4%) in the SRS group took salvage WBRT when CNS lesions progressed after SRS (p=0.67). Among the patients with CNS disease progression, five were considered that benefits would be less than risk by booster WBRT (Table 2). Four patients could not take WBRT due to poor performance, and others refused additional radiation therapy or took other treatment such as intracranial chemotherapy via ommaya reservoir. The median OS of SRS with TKI group was 37.1 months, compared with 31.9 months in the SRS without TKI group (p=0.19). The median PFS time was not significantly longer in the former group than in the latter (17.0 months vs. 9.0 months; p=0.450) (Fig. 2).
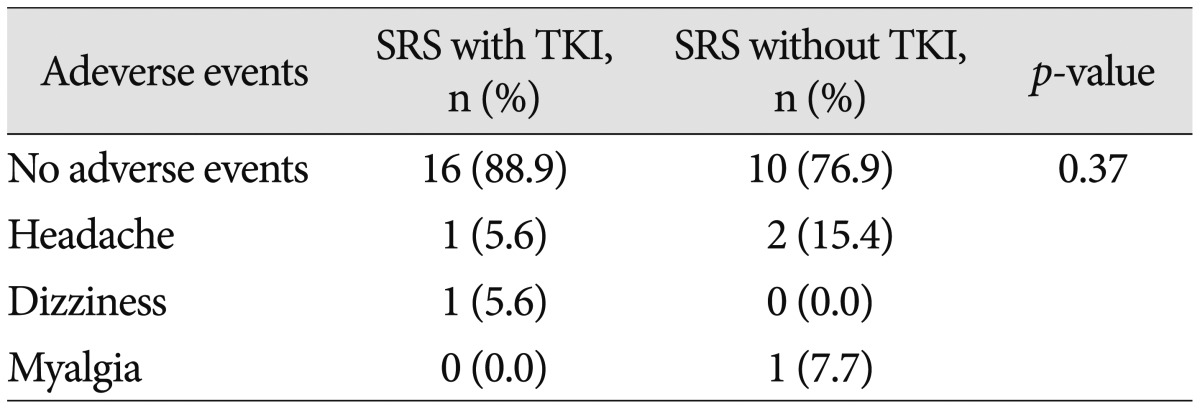
The most common complaint after SRS was headache but this was controlled using acetaminophen. There were no severe adverse events that required additional steroid treatment or surgery following SRS (Table 3). In addition, a combination of SRS and TKI did not induce additional adverse effects. After SRS, two patients experienced dizziness and myalgia, but these symptoms subsided by themselves.
Our present study did not show that adding a usual dose of gefitinib or erlotinib to SRS for brain metastases from NSCLC had an additive benefits compared to SRS monotherapy for brain metastases. There were no additional adverse events with the use of concurrent TKI and SRS for brain metastases from EGFR-mutant adenocarcinomas of the lung. Since patients with symptomatic brain metastases have usually been excluded from the large clinical trials of EGFR-TKI16), the efficacy of these drugs against brain metastases has been evaluated in previous case reports and retrospective small studies621). There is evidence to support that gefitinib has a significant role in brain metastases2817) and the efficacy of high-dose weekly erlotinib on CNS metastases has been described in other reports3411). In those studies, the presence of activating EGFR mutation was a significant predictor for clinical benefit of brain metastases21). However, although all of our current enrolled patients had an activating EGFR mutation, the efficacy of TKI agents on brain metastases was not significantly better than chemotherapy, possibly because these inhibitors were used at the usual dose and the resulting penetration of blood-brain barrier was insufficient to reach a therapeutic concentration in the CNS. Moreover, our number of enrolled patients was limited. Furthermore, our present study included diverse brain metastases settings : brain metastases at first diagnosis of NSCLC, brain metastases relapse after curative surgery, and new development of brain metastases on systemic therapy. The effect of systemic treatment could vary according to these various clinical settings.
In recent investigations, SRS with booster WBRT has shown advantages in the local control of brain metastases110). In addition, concomitant treatment of WBRT with gefitinib has shown promising activity in a Chinese population with regard to efficacy and adverse effects13). Based on these results, EGFR-TKI and SRS with adjuvant WBRT could be an option for the limited number of EGFR-mutant NSCLC patients with brain metastases. However, a well-designed randomized control trial is necessary to establish an effective treatment strategy for EGFR-mutant NSCLC patients with brain metastases.
Patients with activating EGFR mutations have significantly better survival outcomes than those with wild-type EGFR when treated with EGFR-TKIs1524). Despite brain metastases, the median OS time of the enrolled patients in this study was 23.1 months. As the survival time of advanced NSCLC patients with activating EGFR mutations has lengthened due to the development of targeted therapies, treatment strategies for brain metastases need to improve to a similar extent. Nowadays, as early brain metastases without symptoms and signs can be detected by advanced imaging techniques, the indications for SRS have been increasing. SRS could avoid the risk of neurocognitive impairment and a decline in quality of life associated with WBRT and can be applied to elderly patients without severe adverse effects14). According to the study enrolled NSCLC patients with brain metastases harboring an activating EGFR mutation without neurologic symptom, first line EGFR-TKI monotherapy showed that disease control rate and PFS were 93% and 6.6 months, respectively20). In this study, those of SRS monotherapy were 61.5% and 9.0 months. Due to its retrospective nature, our current study has several limitations. First, because the guideline recommendations were changed in 2010 in Korea, with EGFR-TKI changed from a second- to first-line treatment, our study patients had different treatment settings. Recently, EGFR-TKI has been used as a first-line treatment for patients harboring an activating EGFR mutation, and it is now recommended to continue EGFR-TKI treatment for lung cancer patients with advanced cancers, even if brain metastases have newly developed18). Second, different chemotherapy regimens were used among our patient series. Although all of the chemotherapy regimens used in this study included a standard treatment for NSCLC, these regimens were still variable. Third, there was no tissue confirmation of brain metastases in our subjects. When there was a recurrence of brain metastases, a re-biopsy was not performed and the metastases were mainly diagnosed by radiologic results. Also, without the availability of brain tissue, the EGFR mutation status was determined using previous lung tissue. Finally, the EGFR-TKI used was either gefitinib or erlotinib. Both drugs showed treatment efficacy against brain metastases5920), but the characteristics of these drugs and interactions with other medications such as anticonvulsants and steroids could be different. EGFR mutation is more common in Korea than other countries, and overall survival of the patients with EGFR mutation has become improved. Therefore, there is a great need to establish proper treatment strategies for the patients with EGFR-mutant brain metastases.
This present study showed no additive benefit of a combination therapy of SRS and EGFR-TKI for brain metastases from EGFR-mutant adnocarcinomas of the lung. Further investigation including an prospective study is needed to determine the efficacy of an EGFR-TKI add-on in treating this subgroup of NSCLC patients with SRS.
References
1. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases : a randomized controlled trial. JAMA. 2006; 295:2483–2491. PMID: 16757720.

2. Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crinò L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer : a prospective trial. Ann Oncol. 2004; 15:1042–1047. PMID: 15205197.

3. Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010; 99:283–286. PMID: 20146086.

4. Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011; 13:1364–1369. PMID: 21865399.

5. Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010; 16:5873–5882. PMID: 21030498.

6. Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004; 46:255–261. PMID: 15474674.

7. Kim HS, Koh EJ, Choi HY. Multiple gamma knife radiosurgery for multiple metachronous brain metastases associated with lung cancer : survival time. J Korean Neurosurg Soc. 2012; 52:334–338. PMID: 23133721.

8. Kim MK, Lee KH, Lee JK, Choi JH, Hyun MS. Gefitinib is also active for carcinomatous meningitis in NSCLC. Lung Cancer. 2005; 50:265–269. PMID: 16024135.

9. Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim SW, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer. 2012; 75:82–88. PMID: 21684626.

10. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases : results of the EORTC 22952-26001 study. J Clin Oncol. 2011; 29:134–141. PMID: 21041710.

11. Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases--one with a remarkable thoracic response as well. Lung Cancer. 2013; 80:102–105. PMID: 23375403.

12. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005; 23:6207–6219. PMID: 16135488.

13. Ma S, Xu Y, Deng Q, Yu X. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. 2009; 65:198–203. PMID: 19091441.

14. Minniti G, Esposito V, Clarke E, Scaringi C, Bozzao A, Lanzetta G, et al. Stereotactic radiosurgery in elderly patients with brain metastases. J Neurooncol. 2013; 111:319–325. PMID: 23187817.

15. Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005; 23:2513–2520. PMID: 15738541.

16. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405) : an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128. PMID: 20022809.

17. Namba Y, Kijima T, Yokota S, Niinaka M, Kawamura S, Iwasaki T, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer : review of 15 clinical cases. Clin Lung Cancer. 2004; 6:123–128. PMID: 15476598.

18. National Comprehensive Cancer Network I. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Non-Small Cell lung cancer. Washington DC: National Comprehensive Cancer Network;2014.
19. Park BJ, Lim YJ, Kim TS, Rhee BA, Leem W, Kim GK. Brain metastasis from lung cancer-treatment and prognosis-. J Korean Neurosurg Soc. 1998; 27:53–58.
20. Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012; 77:556–560. PMID: 22677429.

21. Porta R, Sánchez-Torres JM, Paz-Ares L, Massutí B, Reguart N, Mayo C, et al. Brain metastases from lung cancer responding to erlotinib : the importance of EGFR mutation. Eur Respir J. 2011; 37:624–631. PMID: 20595147.

22. Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for non-small cell lung carcinoma metastatic to the brain : long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002; 97:1276–1281. PMID: 12507123.

23. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29. PMID: 24399786.

24. Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005; 23:6829–6837. PMID: 15998907.

25. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.
26. Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperriere N, et al. Radiotherapeutic management of brain metastases : a systematic review and meta-analysis. Cancer Treat Rev. 2005; 31:256–273. PMID: 15951117.

27. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013; 31:895–902. PMID: 23341526.

Fig. 1
Flow chart of enrolled patients. SRS : stereotactic radiosurgery, BM : brain metastasis, NSCLC : non-small-cell lung cancer, EGFR : epithelial growth factor receptor, TKI : tyrosine kinase inhibitor.
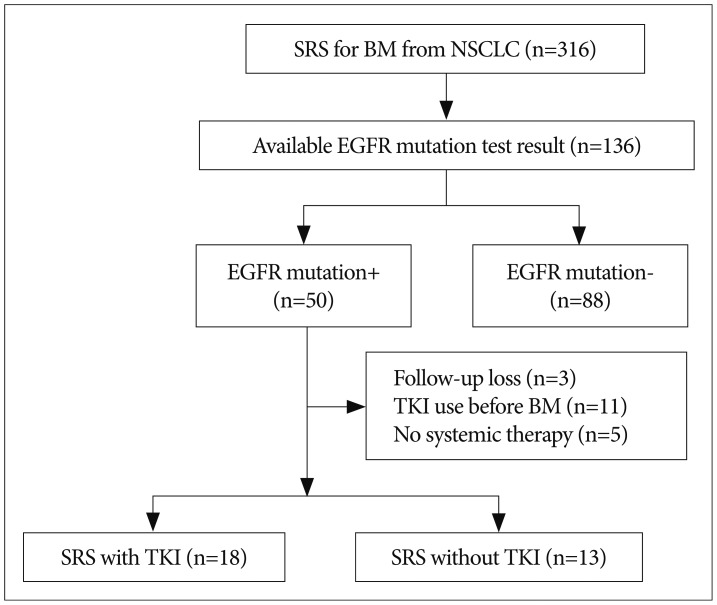
Fig. 2
Kaplan-Meier analysis of progression-free survival and overall survival. TKI : tyrosine kinase inhibitor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download