Abstract
Objective
Aneurysms arising from the pericallosal artery (PA) are uncommon and challenging to treat. The aim of this study was to report our experiences of the endovascular treatment of ruptured PA aneurysms.
Methods
From September 2003 to December 2013, 30 ruptured PA aneurysms in 30 patients were treated at our institution via an endovascular approach. Procedural data, clinical and angiographic results were retrospectively reviewed.
Results
Regarding immediate angiographic control, complete occlusion was achieved in 21 (70.0%) patients and near-complete occlusion in 9 (30.0%). Eight procedure-related complications occurred, including intraprocedural rupture and early rebleeding in three each, and thromboembolic event in two. At last follow-up, 18 patients were independent with a modified Rankin Scale (mRS) score of 0-2, and the other 12 were either dependent or had expired (mRS score, 3-6). Adjacent hematoma was found to be associated with an increased risk of poor clinical outcome. Seventeen of 23 surviving patients underwent follow-up conventional angiography (mean, 16.5 months). Results showed stable occlusion in 14 (82.4%), minor recanalization in two (11.8%), and major recanalization, which required recoiling, in one (5.9%).
Conclusion
Our experiences demonstrate that endovascular treatment for a ruptured PA aneurysms is both feasible and effective. However, periprocedural rebleedings were found to occur far more often (20.0%) than is generally suspected and to be associated with preoperative contrast retention. Analysis showed existing adjacent hematoma is predictive of a poor clinical outcome.
Distal anterior cerebral artery aneurysms, also known as pericallosal artery (PA) aneurysms, are located on the anterior cerebral artery distal to the anterior communicating artery. These aneurysms represent approximately 6% of all intracranial aneurysms and 4% of those that rupture49). Furthermore, they were similarly represented in the International Subarachnoid Aneurysm Trial (ISAT) study population, in which they accounted for 95 of the 2143 patients randomized (4.4%)14). PA aneurysms present several problems for direct surgical clipping, that is, difficult exposure via an interhemispheric approach, especially in a swollen brain after subarachnoid hemorrhage (SAH), sacrifice of a bridging vein for adequate surgical exposure (thus increasing the risk of postoperative morbidity), difficult controlling the parent artery, and the unfavorable orientation of the aneurysm fundus. As a consequence, surgical morbidity has been reported to be relatively high, with incidences ranging from 0 to 25%141221). Advances in endovascular techniques and devices have led to the rapid evolution of coil embolization of ruptured and unruptured intracranial aneurysms, and it has now become an efficient treatment technique that is comparable to surgical clipping. However, because of their distal location and the small diameter of the parent vessel, PA aneurysms pose a technical challenge for endovascular therapy and, as is the case for most bifurcation cerebral aneurysms, have a potential high recurrence rate18). A search of the literature revealed relatively few studies have been performed on the endovascular treatment of ruptured PA aneurysms, and that the studies performed involved relatively small numbers of patient. Accordingly, it is evident that the effectiveness and safety of the endovascular treatment of ruptured PA aneurysms have not been well-defined. Here, we present 30 patients that were treated endovascularly for a ruptured PA aneurysm and describe their clinical and radiologic outcomes.
We retrospectively analyzed the angiographic and clinical records of all patients that underwent endovascular treatment for a ruptured PA aneurysm at our institution from September 2003 to December 2013. The treatment strategy for ruptured PA aneurysm at our institution was to attempt endovascular treatment as the first-line treatment. This strategy was applied to those accompanied by intraparenchymal hematoma (ICH), and surgical evacuation, if necessary, was performed in the immediately following coiling. However, microsurgery was used in the early years of this study because the technology for coiling of distally located aneurysms was in its infancy. Therefore, surgical clipping was first attempt in 6 cases during the same period. There was no technical failure in both clipping and coiling groups. Due to the retrospective nature, the present study is exempt in accord with the Institutional Review Board standards of our institution.
Patient demographic data and characteristics of the aneurysms are provided in Table 1. Thirty ruptured PA aneurysms in 30 patients were treated endovascularly, and there were 10 males and 20 females of mean age 55.9 years (range, 24 to 88 years). Dissecting or fusiform aneurysms, aneurysms associated with a brain arteriovenous malformation, and traumatic or mycotic aneurysms were excluded. Size of aneurysms and parent arteries were measured automatically and manually on two-dimen sional digital subtraction angiography working views. Aneurysm size was defined as the maximum distance of the dome from the neck plane. Aneurysm and neck sizes ranged from 2.5 to 10.4 mm (mean 5.0 mm) and from 0.9 to 6.1 mm (mean 2.2 mm), respectively. 21 of the 30 saccular aneurysms were defined as wide-neck aneurysms (a large neck size of ≥4 mm and/or a dome-to-neck ratio of ≤2). Of the 30 PA aneurysms, 23 (76.7%) were located at the pericallosal-callosomarginal junction and 7 (23.3%) at the pericallosal-frontopolar junction. Fifteen PA aneurysms were on the left side, 13 on the right, and 2 at midline (azygos artery). Eight (26.7%) patients had at least one additional aneurysm. All patients were clinically assessed at admission using Hunt and Hess grades; 12 patients (40.0%) grade II, 9 (30.0%) grade III, 6 (20.0%) grade V, and 3 (10.0%) grade IV. Fifteen patients (50.0%) had a concomitant ICH that was managed conservatively in all but two cases that required surgical evacuation.
In general, endovascular treatment was performed as soon as possible after SAH, regardless of clinical condition. No antiplatelet premedication was prescribed in any patients because of the risk of rebleeding. Coiling of aneurysms was performed under local anesthesia, and intravenous bolus injection of standard heparin was not performed during the procedure. However, drip infusion of heparin was carried out employing catheter pressure infusion systems with continuous infusion of 1000 U of heparin per 1000 mL of saline. Two patients, who required ICH removal, were excluded from systemic heparinization.
The aim of the coiling was to obtain a packing of the aneurysm as attenuated as possible. Various technical measures and precautions were taken to overcome specific problems. The remodeling techniques using a balloon or stent or multiple catheters were unavailable for the aneurysms treated during the early part of our series. However, we have recently favored these remodeling techniques to achieve higher postoperative occlusion rates. When an aneurysm could not be reached using standard procedures because of a distal location and instability of the microcatheter, the guiding catheter was placed more distal into the internal carotid artery. Care was taken not to interfere with the flow of the carotid or parent artery. By using these means, all aneurysms were reached with a microcatheter. In the case of intraprocedural thromboembolic complication, various strategies for thrombolysis were applied, such as mechanical thrombolysis and intra-arterial or intravenous abciximab, or heparin infusion during or after the procedure.
Immediately after the procedure, a complete neurological examination was performed on all patients by a vascular neurosurgeon. Furthermore, all patients underwent a non-enhanced brain computed tomography (CT) scan to evaluate possible hemorrhagic complications. Unless the thromboembolic complication occurred or a stent was used, further antiplatelet or anticoagulant medications were not administered. The indications for long-term antiplatelet therapy of acetylsalicylic acid and/or clopidogrel were; the formation of a thrombus at the end of the coil or thromboembolic events, stent-assisted coiling, and coil protrusion into the parent artery.
Immediate and follow-up angiographic results were analyzed. Occlusion was classified as; complete when no contrast filling of the aneurysmal sac and neck was observed, near-complete when contrast filling of the neck of the aneurysm was slow or partial when any degree of contrast filling was observed within the aneurysm sac. Clinical results were assessed upon discharge from hospital or at last visit using the modified Rankin Scale (mRS), as follows : 0, no symptoms at all; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death. Poor clinical outcome was defined as a mRS score of 3 to 6. Complications were defined as all adverse events related to the procedure, and these were studied retrospectively using medical and operative reports. Periprocedural rebleeding contained both intraprocedural rupture and early rebleeding. Intraprocedural rupture was defined as clinically and angiographically evident rupture during the procedure. Early rebleeding was defined as expanding hemorrhage with worsening of the patient's condition within 30 days after coiling, and diagnostic confirmation was made by CT when it showed an increased amount of hemorrhage compared with immediate postprocedural CT.
Univariate and multivariate logistic regression analyses were used to identify risk factors associated with periprocedural rebleeding or a poor clinical outcome. Results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Variables showing a univariate association with a dependent risk factor (p<0.10) were included in the multivariate logistic analysis. All analyses were performed using SPSS version 20.0 SPSS Inc., Chicago, IL, USA). Statistical significance was accepted for p values of <0.05.
Procedural complications and treatment results are summarized in Table 2.
The microcatheter was navigated into the lumen of the PA aneurysm and coils were delivered in all 30 patient. The treatment of 19 aneurysms was straightforward, but the other 11 required an adjunctive technique, that is, double catheter, balloon-assisted, or a stent-assisted technique. Immediate post-procedural angiograms showed complete aneurysm occlusion in 21 (21/30, 70.0%) and near-complete occlusion in 9 (9/30, 30.0%).
Procedure-related thromboembolic complications developed in two patients (2/30, 6.7%). In one patient, occlusion of a callosomarginal artery occurred due to thrombus formation at the artery origin, which resulted in right leg monoparesis. The patient fully recovered over the 1st postoperative year. The other thromboembolism occurred in the inferior M2 segment of the middle cerebral artery during the procedure, a relevant infarction was observed 2 days after coiling. However, the patient concerned did not experience any neurological symptom.
Intraprocedural rupture occurred from three aneurysms (3/30, 10.0%). Diagnosis of intraprocedural rupture was made based on the angiographic visualization of contrast material extravasation in two cases. The coil was observed partially outside the aneurysm with a sudden rise in arterial blood pressure but without angiographic demonstration of extravasation of contrast medium in one case. Early rebleeding after coiling also occurred in three patients (3/30, 10.0%). The mean delay between coiling and early rebleeding was 31 hours (range, 2-83 hours). In these three patients, intravenous bolus of 3000 IU of heparin was injected during the procedure only in one case needed a stent. Besides drip infusion of heparin during the procedure, further antiplatelet or anticoagulant medications were not administered in the other two cases. Of these six patients with periprocedural rebleeding, five had small (≤4 mm) aneurysms, but one had large (9.9 mm) and multi-lobulated aneurysm. Furthermore, the five patients (5/6, 83.3%) concerned had an adjacent hematoma on initial CT scans, and three (3/6, 50.0%) showed contrast retention and delayed washout by the aneurysm by preprocedural digital subtraction angiography. In these six patients with periprocedural rebleeding, no new neurologic deficit developed in three patients (3/6, 50.0%), but deterioration of consciousness, resulting in death, occurred in two patients (2/6, 33.3%). It could be questioned as to whether the early rebleeding had worsened clinical status in one patient, because the patient had initial severe SAH and died within 5 days after SAH onset. Thus, the periprocedural mortality in our cohort was 6.7% (2/30) and morbidity was 3.3% (1/30).
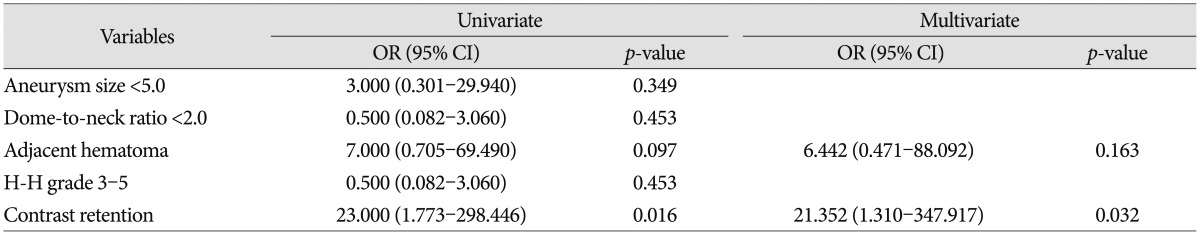
Table 3 provides a summary of the risk factors found to be associated with periprocedural rebleeding. The associations of all variables with periprocedural rebleeding were examined by univariate logistic regression analysis, and contrast retention was found to be associated with an increased risk of periprocedural rebleeding (OR : 23.000; 95% CI : 1.773 to 298.446; p=0.016). Furthermore, multivariate logistic-regression analysis adjusted for adjacent hematoma confirmed the validity of this relationship (OR : 21.352; 95% CI : 1.310 to 347.917; p=0.032).
Only one patient (1/30, 3.3%) suffered from symptomatic vasospasm and, fortunately, no negative long-term effects were observed. Two patients (2/30, 6.7%) suffered from shunt-dependent hydrocephalus. Clinical results were assessed upon discharge from hospital or at last follow-up visit using the mRS. At the end of the observational period, 18 patients were independent with a mRS score of 0-2 (18/30, 60.0%), and the other 12 (12/30, 40%) were dependent (mRS score, 3-6). Seven of the 12 had a mRS score of 6 (due to a poor preoperative status in five and procedure-related rebleeding in two), three had a mRS score of 5 (due to poor preoperative status or old age), one had a mRS score of 4 (due to old age), and one had a mRS score of 3 (due to a large ICH). The 23 surviving patients were followed for 6-68 months (mean, 32.7 months). No neurologic deterioration or hemorrhagic complication occurred during follow-up period in any of these patients.
Predictors of a poor clinical outcome were analyzed (Table 4). Univariate logistic-regression analysis showed that age (OR : 1.080; 95% CI : 1.003 to 1.163; p=0.043), adjacent hematoma (OR : 13.000; 95% CI : 2.074 to 81.479; p=0.006), and Hunt and Hess grade 3-5 (OR : 13.750; 95% CI : 1.452 to 130.239; p=0.022) were associated with a poor clinical outcome. However, multivariate logistic-regression analysis adjusted for age and Hunt and Hess grade 3-5 showed that only adjacent hematoma was associated with a poor clinical outcome (OR : 47.734; 95% CI : 1.596 to 1427.240; p=0.026).
Seventeen of the 23 surviving patients underwent follow-up conventional angiography between 1 and 65 months postoperatively (mean, 16.5 months). According to the follow-up images obtained, 9 of the 10 initially occluded aneurysms remained completely occluded and the remaining one showed partial recanalization due to coil compaction. Repeat follow-up angiography at 20 months showed in this patient that the condition was stable, and the patient concerned declined additional therapy. Five of the 7 aneurysms initially showing near complete occlusion remained stable at follow-up. The other two showed minor recanalization and major recanalization, respectively. Recoiling was undertaken in the patient showing major recanalization at 13 months postoperatively, and complete occlusion was achieved. Thus, the overall stable occlusion rate was 82.4% (14/17) and the recanalization rate was 17.6% (3/17). Parent arteries of the aneurysm treated by Y-stent-assisted coiling remained patent with no evidence of intimal hyperplasia or in-stent stenosis. Representative cases are shown in Fig. 1, 2.
Ruptured PA aneurysms may be technically difficult to clip because of the narrow exposure afforded by an interhemispheric approach, difficulty control of the parent artery, and sacrifice of a bridging vein to achieve adequate surgical exposure. Furthermore, many patients suffer intra-operative hemorrhage. As a consequence, surgical morbidity has been reported to be relatively high, with incidences ranging from 0 to 25%141221).
Endovascular treatment of ruptured PA aneurysms has been reported to be technically difficult, primarily because of the long access route, which complicates microcatheter manipulation, and the small aneurysmal lumen. The introduction of new microcatheter and microguidewire with improved trackability, pushability, and torque and novel hydrophilic coatings have facilitated navigation into distant cerebral arteries, such as, the PA. Furthermore, the availabilities of various soft coils may have contributed to the higher success rate of small aneurysm coiling without intraoperative rupture. We found that the PA could be reached and coiling of the aneurysm accomplished in all 30 patients. In fact, even a 2.5 mm sized aneurysm located at a distal junction was occluded successfully.
In our series, an independent daily living activity level (mRS score, 0-2) was achieved by 60% of our patients (18/30). The poor clinical outcomes (mRS score, 3-6) of the other 12 patients resulted from poor preoperative status, periprocedural rebleeding, old age, and large ICH. Waldenberger et al.25) reported that the most important negative contributor to the outcome of PA aneurysm coiling was the presence of ICH, and this was followed by thromboembolic complications and poor preoperative status. Ruptured PA aneurysms cause ICH, in addition to SAH, in more than one-half of cases, and are associated with poorer outcomes after rupture than aneurysms in other locations311). In the present study, 15 of the 30 patients (50.0%) had a concomitant ICH that was visualized on initial CT scans, and 10 of the patients (10/15, 66.7%) had a poor clinical outcome (mRS score, 3-6). Furthermore, multivariate logistic-regression analysis showed that adjacent hematoma was the only independent predictor of a poor clinical outcome (OR : 47.734; 95% CI : 1.596 to 1427.240; p=0.026). Other variables, such as, age and Hunt and Hess grade were of borderline significance, possibly due to the small number of patients enrolled (Table 4).
We have summarized the reported studies in which more than 10 patients with ruptured pericallosal artery aneurysms were treated with endovascular treatment in Table 5. In a recently reported meta-analysis of endovascular treatment of PA aneurysms including 16 studies, the authors concluded that endovascular treatment of PA aneurysms is associated with high angiographic occlusion rates, but the complication rates are higher compared with other aneurysms in the circle of Willis. The overall rate of operative complications was 12%. Procedure-related ischemia and iatrogenic rupture occurred in 5% and 7% of patients, respectively. Procedure-related permanent morbidity rate was 8%23).
In the present study, the overall procedure-related complication rate was 26.7% (8/30 cases). Two patients experienced thromboembolic events and acute infarctions and developed corresponding neurologic deficits, though fortunately showed no neurological deficit at follow-up. Intraprocedural rupture occurred from three aneurysms (10.0%), which was markedly greater than anticipated. A review of the literature revealed PA aneurysms have a higher procedure-related rupture rate than non-PA aneurysms. Keston et al.9) reported an intraprocedural rupture rate of 18% (3/17 patients) for PA aneurysms, which compared with an overall departmental five year (1999-2003 inclusive) intraprocedural rupture rate of only 1.1% (7/640 patients) for non-PA aneurysms. Nguyen et al.15) reported a 12% (3/25 patients) procedure-related perforation rate in their endovascular series of PA aneurysms. Based on these reports and the fact that previous reports included unruptured cases, whereas we did not, we believe that our intraprocedural rupture rate is not excessive. It is well known PA aneurysms are smaller than non-PA aneurysms tend and that they tend to rupture at a smaller size101327). In a study of a large number of aneurysm cases, the average diameter of ruptured aneurysms was reported to be 8.2 mm8), whereas the average aneurysm diameter in our patients was 5.0 mm. We believe this difference could explain the higher intraprocedural rupture rate in the endovascular treatment of ruptured PA aneurysms. In addition, to aneurysm size, the risk of procedure-induced perforation may also be related to anatomical peculiarities. A more distal location leads to catheter positioning via a longer segment in a small-caliber vessel, which reduces the degree of catheter deflection when a coil encounters resistance15).
Prior studies on early rebleeding after coiling have reported rupture rates of 1.4% to 2.6%6722). Incomplete embolization, adjacent hematoma, small aneurysms, and post-procedural anticoagulation have been reported to be associated with early rebleeding after coiling. The Cerebral Aneurysm Rerupture After Treatment study reported that the risk of posttreatment rebleeding was closely associated with the degree of aneurysm occlusion7). However, we experienced three cases with early rebleeding after coiling (10.0%), despite all three aneurysms being complete embolized during the procedure. The presence of adjacent hematoma is a strong, independent risk factor of early rebleeding and a thrombosed pseudoaneurysm and hematoma could cause early reopening and rehemorrhage22). In our series, all three cases with early rebleeding accompanied by adjacent hematoma. At coiling, a pseudoaneurysm may or may not be visible by angiography. If a pseudoaneurysm is apparent by angiography, coiling of the true aneurysm and the pseudoaneurysm can be considered, but the fragile wall of the pseudoaneurysm is prone to perforation, and this has been prompted to advise against catheterization into false lumen16). On the other hand, Cho et al.2), who reported on early recurrent hemorrhage after coil embolization in ruptured intracranial aneurysms, hypothesized another possibility that rebleeding with ICH may be caused by delayed hemorrhage or propagation of the initial ICH due to vulnerability of the parenchyma adjacent to the ICH, rather than by rebleeding of the coiled aneurysm. Early rebleeding after coiling of a ruptured aneurysm and intra-procedural rupture are major concerns, because associated mortality rates are high. When a ruptured PA aneurysm accompanied by adjacent hematoma, decisions regarding endovascular treatment should be taken only after carefully considering the possibility of periprocedural rebleeding. Therefore, it may be better to consider the surgical clipping of a ruptured PA aneurysm with adjacent hematoma as a first option to prevent periprocedural rebleeding.
With regard to treatment durability, recurrence requiring recoiling due to the risk of re-rupture, developed in one of our 17 cases, who developed major recanalization. In the present study, the overall recanalization rate was 17.6% (3/17) but the major recanalization rate was only 5.9% (1/17). Recurrence after coiling has been reported to be associated primarily with aneurysm size7162226). In one study, follow-up angiographic studies after complete occlusion showed recurrence rates of 14% for aneurysms of <5 mm, 27% for aneurysms of 5-10 mm, and 57% for aneurysms of ≥10 mm23). Therefore, the lower rate of recurrence in our series could have been due to the lower proportion of cases with aneurysms ≥5 mm (9/30, 30.0%). However, in a recent series of 16 cases of PA aneurysm coiling, there was a comparatively high recurrence rate (one major and five minor recurrences, 37.5%), attributed to the loose coil packing rate19).
This study is limited by its retrospective design, patient-selection bias, small cohort, relatively short angiographic follow-up, by the fact that it was conducted at a single institution. Nonetheless, our findings suggest that the endovascular treatment of ruptured PA aneurysms appears to be a valid treatment strategy given suitable levels of institutional and operator expertise.
Our experiences show that the endovascular treatment of ruptured PA aneurysms is both feasible and effective. However, they show periprocedural rebleeding occur far more frequently (20.0%) than is generally considered and that these rebleeding are associated with preprocedural contrast retention. Accordingly, our findings caution that decisions regarding endovascular treatment in cases showing preprocedural contrast retention should approached by carefully considering the possibility of periprocedural rebleeding. Furthermore, an existing adjacent hematoma was found to have a severe negative influence on clinical outcome.
Acknowledgements
This work was supported by clinical research grant from Pusan National University Hospital 2012.
References
1. Chalif DJ, Weinberg JS. Surgical treatment of aneurysms of the anterior cerebral artery. Neurosurg Clin N Am. 1998; 9:797–821. PMID: 9738108.

2. Cho YD, Lee JY, Seo JH, Kang HS, Kim JE, Kwon OK, et al. Early recurrent hemorrhage after coil embolization in ruptured intracranial aneurysms. Neuroradiology. 2012; 54:719–726. PMID: 21969241.

3. de Sousa AA, Dantas FL, de Cardoso GT, Costa BS. Distal anterior cerebral artery aneurysms. Surg Neurol. 1999; 52:128–135. discussion 135-136PMID: 10447278.

4. Hernesniemi J, Tapaninaho A, Vapalahti M, Niskanen M, Kari A, Luukkonen M. Saccular aneurysms of the distal anterior cerebral artery and its branches. Neurosurgery. 1992; 31:994–998. discussion 998-999PMID: 1470334.

5. Huang Q, Shen J, Xu Y, Liu J. Endovascular treatment of ruptured distal anterior cerebral artery aneurysm. Neurol India. 2010; 58:259–263. PMID: 20508346.

6. Jartti P, Isokangas JM, Karttunen A, Jartti A, Haapea M, Koskelainen T, et al. Early rebleeding after coiling of ruptured intracranial aneurysms. Acta Radiol. 2010; 51:1043–1049. PMID: 20849318.

7. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR, et al. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms : the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. 2008; 39:120–125. PMID: 18048860.

8. Kassell NF, Torner JC. Size of intracranial aneurysms. Neurosurgery. 1983; 12:291–297. PMID: 6843800.

9. Keston P, White PM, Horribine L, Sellar R. The endovascular management of pericallosal artery aneurysms. J Neuroradiol. 2004; 31:384–390. PMID: 15687957.

10. Lee JW, Lee KC, Kim YB, Huh SK. Surgery for distal anterior cerebral artery aneurysms. Surg Neurol. 2008; 70:153–159. discussion 159PMID: 18482760.

11. Lehecka M, Lehto H, Niemelä M, Juvela S, Dashti R, Koivisto T, et al. Distal anterior cerebral artery aneurysms : treatment and outcome analysis of 501 patients. Neurosurgery. 2008; 62:590–601. discussion 590-601PMID: 18425008.
12. Martines F, Blundo C, Chiappetta F. Surgical treatment of the distal anterior cerebral artery aneurysms. J Neurosurg Sci. 1996; 40:189–194. PMID: 9165426.
13. Menovsky T, van Rooij WJ, Sluzewski M, Wijnalda D. Coiling of ruptured pericallosal artery aneurysms. Neurosurgery. 2002; 50:11–14. discussion 14-15PMID: 11844229.

14. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms : a randomised trial. Lancet. 2002; 360:1267–1274. PMID: 12414200.

15. Nguyen TN, Raymond J, Roy D, Chagnon M, Weill A, Iancu-Gontard D, et al. Endovascular treatment of pericallosal aneurysms. J Neurosurg. 2007; 107:973–976. PMID: 17977269.

16. Nomura M, Kida S, Uchiyama N, Yamashima T, Yoshikawa J, Yamashita J, et al. Ruptured irregularly shaped aneurysms : pseudoaneurysm formation in a thrombus located at the rupture site. J Neurosurg. 2000; 93:998–1002. PMID: 11117874.

17. Oishi H, Nonaka S, Yamamoto M, Arai H. Feasibility and efficacy of endovascular therapy for ruptured distal anterior cerebral artery aneurysms. Neurol Med Chir (Tokyo). 2013; 53:304–309. PMID: 23708221.

18. Pandey A, Rosenwasser RH, Veznedaroglu E. Management of distal anterior cerebral artery aneurysms : a single institution retrospective analysis (1997-2005). Neurosurgery. 2007; 61:909–916. discussion 916-917PMID: 18091267.
19. Park HS, Kwon SC, Kim MH, Park ES, Sim HB, Lyo IU. Endovascular coil embolization of distal anterior cerebral artery aneurysms : angiographic and clinical follow-up results. Neurointervention. 2013; 8:87–91. PMID: 24024072.

20. Park KY, Kim BM, Lim YC, Chung J, Kim DJ, Joo JY, et al. The role of endovascular treatment for ruptured distal anterior cerebral artery aneurysms : comparison with microsurgical clipping. J Neuroimaging. 2015; 25:81–86. PMID: 24299470.

21. Proust F, Toussaint P, Hannequin D, Rabenenoïna C, Le Gars D, Fréger P. Outcome in 43 patients with distal anterior cerebral artery aneurysms. Stroke. 1997; 28:2405–2409. PMID: 9412622.

22. Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms : incidence, morbidity, and risk factors. AJNR Am J Neuroradiol. 2005; 26:1739–1743. PMID: 16091523.
23. Sturiale CL, Brinjikji W, Murad MH, Cloft HJ, Kallmes DF, Lanzino G. Endovascular treatment of distal anterior cerebral artery aneurysms : single-center experience and a systematic review. AJNR Am J Neuroradiol. 2013; 34:2317–2320. PMID: 23868164.

24. Suzuki S, Kurata A, Yamada M, Iwamoto K, Nakahara K, Sato K, et al. Outcomes analysis of ruptured distal anterior cerebral artery aneurysms treated by endosaccular embolization and surgical clipping. Interv Neuroradiol. 2011; 17:49–57. PMID: 21561558.

25. Waldenberger P, Petersen J, Chemelli A, Schenk C, Gruber I, Strasak A, et al. Endovascular therapy of distal anterior cerebral artery aneurysms-an effective treatment option. Surg Neurol. 2008; 70:368–377. PMID: 18291498.

26. Xavier AR, Abdelbaky A, Rayes M, Tiwari A, Narayanan S. Clinical and angiographic outcome in patients with completely occluded intracranial aneurysms by endovascular coiling : our experience. J Neurointerv Surg. 2011; 3:335–339. PMID: 21990435.

27. Yamazaki T, Sonobe M, Kato N, Kasuya H, Ikeda G, Nakamura K, et al. Endovascular coiling as the first treatment strategy for ruptured pericallosal artery aneurysms : results, complications, and follow up. Neurol Med Chir (Tokyo). 2013; 53:409–417. PMID: 23803620.

Fig. 1
Images showing a ruptured aneurysm of the right pericallosal artery (PA). A : Unenhanced computed tomography (CT) in a 52-year-old female patient demonstrates diffuse subarachnoid hemorrhage and a scant intraparenchymal hematoma (arrow) at the right cingulate gyrus. B : Conventional angiograph showing a ruptured 9.9 mm PA aneurysm with multiple lobulations. C : Conventional angiogram showing contrast retention and delayed washout (arrow)-a characteristic angiographic feature of cerebral pseudoaneurysm. D and E : Unsubtracted and subtracted images acquired immediately after stent-assisted coiling demonstrating complete aneurysm occlusion. Coiling was uneventful without clinically and angiographically evident intraprocedural rupture. F : Unenhanced CT image obtained 2 hours after the procedure shows hematoma enlargement with worsening of the patient's condition, indicating early rebleeding.
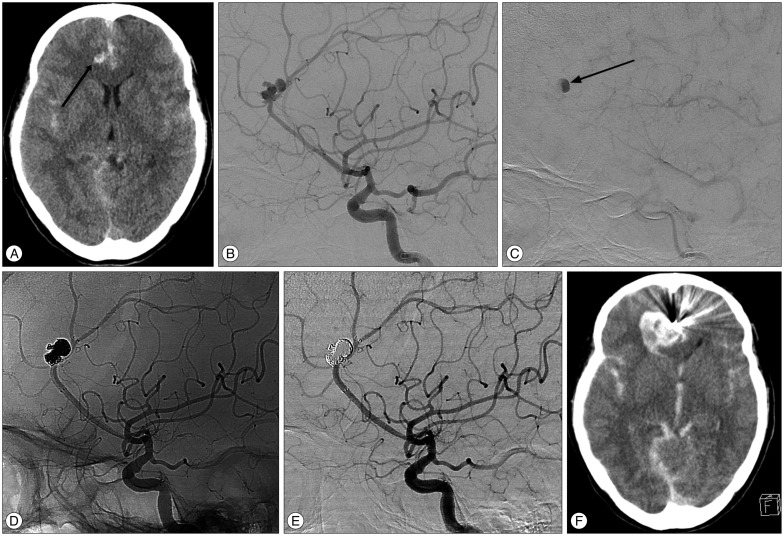
Fig. 2
This 47-year-old female patient of Hunt and Hess grade II was admitted after rupture of a left pericallosal artery aneurysm. A : Unenhanced CT image obtained at admission demonstrated a subtle subarachnoid hemorrhage at the interhemispheric fissure. B : Conventional angiogram shows a ruptured 3.2 mm aneurysm at the pericallosal-frontopolar artery bifurcation. C : A sudden rise of arterial blood pressure occurred during insertion of the 1st coil, indicating rebleeding. Outpouching of part of the coil is seen outside the aneurismal cavity, and 30 mg of protamine sulfate promptly injected. D and E : Unsubtracted and subtracted images obtained immediately after additional coil insertion show extravasation of contrast medium, indicating rupture. F and G : Unsubtracted and subtracted postembolization angiogram (after placement of additional coils) reveals complete aneurysm occlusion and no more extravasation of contrast medium. H : Unenhanced CT image obtained immediately after the procedure showing extravasation of contrast medium and blood in the subarachnoid space and ventricle, indicating rebleeding.
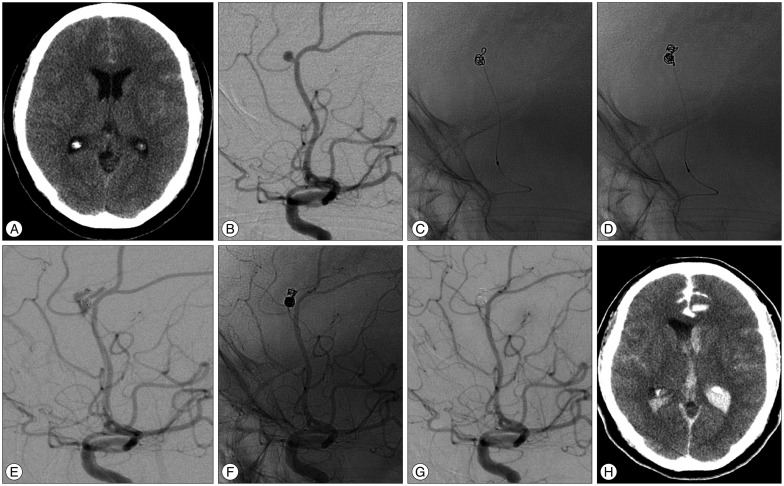
Table 3
Univariate and multivariate analyses of risk factors associated with periprocedural rebleeding




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download