Abstract
Objective
The aim of this study is to investigate good prognostic factors for an acute occlusion of a major cerebral artery using mechanical thrombectomy.
Methods
Between January 2013 to December 2014, 37 consecutive patients with acute occlusion of a major cerebral artery treated by mechanical thrombectomy with stent retrievers were conducted. We analyzed clinical and angiographic factors retrospectively. The collateral flow and the result of recanalization were sorted by grading systems. Outcome was assessed by National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) at 90 days. We compared the various parameters between good and poor angiographic and clinical results.
Results
Twenty seven patients demonstrated good recanalization [Thrombolysis in Cerebral Infarction (TICI) 2b or 3] after thrombectomy. At the 90-day follow up, 19 patients had good (mRS, 0-2), 14 had moderate (3-4) and four had poor outcomes (5-6). The mRS of older patients (≥75 years) were poor than younger patients. Early recanalization, high Thrombolysis in Myocardial Infarction risk score, and low baseline NIHSS were closely related to 90-day mRS, whereas high TICI was related to both mRS and the decrease in the NIHSS.
Acute thromboembolic occlusion of major cerebral arteries is a very urgent situation and requires immediate reperfusion therapy. Endovascular intervention offers promise in patients presenting beyond the 4.5-hr time window16), as well as in <4.5-hr, who are ineligible for tissue plasminogen activator (tPA) or fail to respond to intravenous tPA1921). A thrombolytic agent (urokinase, abciximab, or tirofiban) was used in the past although frequent fatal complications such as huge intracerebral hemorrhage (ICH)192324).
Many studies have reported high recanalization and low complication rates following mechanical thromboembolectomy using a stent retriever system410172122). Although the introduction of mechanical recanalization for acute ischemic stroke is popular, few data are available about the prognostic factors of good outcome from the procedure. The prognostic factors identified could suggest proper decision-making before performing thrombectomy.
We collected data from 37 consecutive patients with acute ischemic stroke who were treated with the Solitaire AB/FR stent device and analyzed the angiographic and clinical factors correlated with good outcomes.
The study was approved by the Institutional Review Board of our university (OC14RISE0070). Data in a prospectively maintained interventional data base at the Stroke Center of our hospital were analyzed for the 24 months since January 2013.
The policies of our Stroke Center for endovascular recanalization are 1) baseline National Institutes of Health Stroke Scale (NIHSS) score ≥4, except for isolated aphasia or hemianopsia, 2) exclusion of hemorrhage by cranial computed tomography (CT) or magnetic resonance imaging (MRI), 3) large vessel [intracranial internal carotid artery (ICA), A1, M1-2, vertebrobasilar artery] occlusion, 4) treatment initiation within 8 hr of symptom onset for hemispheric stroke and within 12 hr for vertebrobasilar stroke, and 5) perfusion-diffusion mismatching confirmed by physician.
All patients within 4.5-hr from symptom treated IV tPA before endovascular procedure. However, we performed endovascular thrombectomy immediately after confirm of large artery occlusion regardless of tPA. NIHSS divided as mild (<10), moderate (10-20), and severe (>20) neurologic deficit.
All endovascular procedures were performed under local anesthesia with proper sedation. All patients treated in our department by intracranial mechanical recanalization using the Solitaire (EV3, Irvine, CA, USA) retrievable stent for acute cerebral artery occlusion were included. Some patients underwent additional balloon angioplasty or permanent stent deployment when recanalization failed.
Collateral flow was graded based on the Thrombolysis in Myocardial Infarction (TIMI) risk score proposed by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology12) : Grade 0, no collateral visible to the ischemic site; grade 1, slow collaterals to the periphery of some of the defect; grade 2, rapid collaterals to the periphery of the ischemic site with persistence of some defect and to only a portion of the ischemic territory; grade 3, collaterals with slow but complete angiographic blood flow to the ischemic bed occurring by the late venous phase; grade 4, complete and rapid collateral blood flow to the vascular bed of the entire ischemic territory, by retrograde perfusion.
The recanalization result was assessed by digital subtraction angiography immediately after the procedure according to Thrombolysis in Cerebral Infarction (TICI) criteria12) : Grade 0, no perfusion; grade 1, penetration with minimal perfusion; grade 2, partial perfusion, subdivided as partial (2a) and complete (2b) filling of entire vascular territory; and grade 3, no flow constraint and complete perfusion. After the thrombectomy, dissection or vessel perforation were assessed carefully.
All patients underwent CT immediately after the procedure to check hemorrhagic transformation. Systolic blood pressure was targeted to 100-120 mm Hg immediately after recanalization using a continuous nicardipine injection to prevent hyperperfusion injury. CT angiography and perfusion CT were performed within 72 hr after the intervention. The occurrence of symptomatic intracerebral hemorrhage was defined as clinical deterioration (≥4-points increase on the NIHSS or 1-point deterioration in the level of consciousness) combined with a space-occupying brain hematoma on a CT scan or MRI.
Clinical outcome was evaluated with the 90-day modified Rankin Scale (mRS) score, the NIHSS, and 90-day mortality. We defined improvement of NIHSS (NIHSS gap) as markedly (≥5) and slightly (<5) decrease. Good long-term outcome was defined as mRS of 0-2, moderate outcome as 3-4 and poor outcome as 5-6 at 3 months.
For categorical variables, the comparisons between good and poor outcome, classified by angiographic and clinical results, were made by using the χ2 test or Fisher exact test if the χ2 was not valid. For continuous variables, the comparisons of means were performed by using a Student t-test. All analyses were performed by using SPSS for Windows, ver. 12.0 (SPSS Inc., Chicago, IL, USA). A p<0.05 was considered significant.
Thirty-seven patients (26 males and 11 females; mean age, 67.38 yr; range, 45-88 yr) with acute ischemic stroke were treated at our institution during the 24 months, and occlusion of an intracranial vessel [distal ICA, anterior cerebral artery (ACA), middle cerebral artery [MCA], or vertebra-basilar artery (VBA)] confirmed by diffusion MRI and CT angiography. Of the 37 patients, most common occlusion sites were MCA (n=22). Distal ICA occlusions were 8, VBA were 6 and ACA was 1. The mean time interval from stroke onset to visiting the hospital was 204±99 min. Mean initial NIHSS score was 16.59±4.83 (range, 8-26) min. After the initial CT, four patients were treated with intravenous tPA (0.9 mg/kg) without clinical improvement. Intravenous administration of tPA was halted when recanalization was achieved. Mean procedure time and symptom to recanalization time were 36.78±16.52 and 241.08±102.24 min respectively.
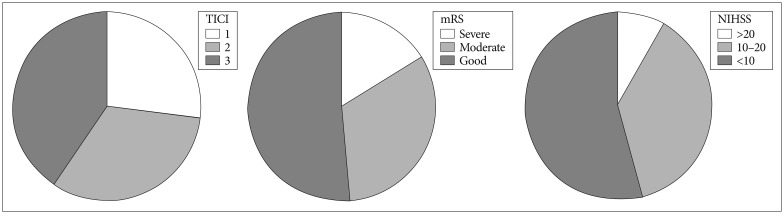
Mean NIHSS at 3 month was 9.45±6.15 (range 2-22). Fig. 1 shows the angiographic and clinical outcome after recanalization. Twenty seven (72.9%) of the 31 patients achieved successful recanalization of TICI grade 2b or 3 after stent retrieval. Mean time taken from femoral puncture to first recanalization was 36.78 min. Seven patients failed recanalization although three thrombectomies with retrievable stents and balloon angioplasty was done. The location of combined severe stenosis was the distal ICA (C6) in three cases, M1 in three cases, and VBA in one case. Three patients achieved recanalization after balloon angioplasty, although the other three patients needed permanent stent deployment for successful recanalization. The other patient, who underwent recanalization of a distal M1-proximal M2 occlusion, failed due to a M2 perforation during selection of the M2 branch with a microwire.
Brain CT scans within 72 hr post-treatment demonstrated no space occupying the intracerebral hematoma except one case of arterial (proximal M2) perforation. We had two mortalities during the study period. One patient died due to an iatrogenic MCA perforation during microwire selection. The other patient died due to a progressive hemispheric infarction and severe brain edema after successful recanalization.
Most patients with a successful recanalization showed immediate clinical symptom improvement. 32 (86.5%) of 37 patients who achieved recanalization showed a decrease of >3 points on the NIHSS in 3 days and 16 of them decreased >5 points. No patient showed re-occlusion or severe stenosis (>70% a normal vessel) after successful recanalization (TICI 2b or 3) by the 90-day follow up. Nineteen patients had good outcomes (mRS 0-2), 14 patients had moderate outcomes (mRS 3-4), and four patients had poor outcomes (mRS 5-6) at the 3 months follow-up.
Table 1 shows the clinical and neuroimaging outcomes. Age, sex, or use of tPA was not related to the decrease in the NIHSS and mRS at 3 months. Elapsed time from symptom onset to recanalization, TIMI, and baseline NIHSS were closely related only to mRS at 90-days, whereas TICI was closely related to both the NIHSS gap and mRS. Younger patients (<75 years) showed better mRS than older (≥75 years) although there was no significant difference in NIHSS gap.
Recanalization of an acute cerebral artery occlusion is a procedure that remarkably improves patient quality of life9). However, previous randomized controlled studies did not prove the superiority of mechanical thrombectomy5713) although recent studies show good results. Early recanalization is the most important factor for a good prognosis141017). However, there few studies have indicated whether a "good prognosis" means remarkable improvement of symptoms (decrease on the NIHSS) or self-reliance in daily life. A remarkable increase on the NIHSS does not always indicate a good quality of life21125).
Most recanalization procedures in the last century were performed through intra-venous or intra-arterial chemical thrombolysis1923). Many patients did not achieve successful recanalization within a measurable time or suffered serious complications such as intracranial hemorrhage, whereas others had a good prognosis. Mechanical thrombectomy (or thromboembolectomy) is the currently used method, and many studies have shown the good results of early recanalization with low complication rates. However, most studies have reported only good results from mechanical thrombectomy, and few studies have been conducted on the factors that influence a good prognosis, although there has been a change in recanalization methods recently314).
In our consecutive case review series using only the Solitaire stent, we achieved a revascularization rate of 73% (TICI 2b or 3), and complete revascularization was achieved in 60% (TICI 3). The mean time taken from femoral puncture to recanalization was 36 min (range, 20-62 min), which is only half or one-third the time compared with previous intra-arterial chemical thrombolysis procedure.
In this study, the outcome of younger patients (<75 years) were significant better than older patients at 3 months although there was no significant difference of NIHSS gap. Short term functional recovery after successful recanalization was similar in all age group. However, older patients are more vulnerable to endovascular procedures due to advanced atherosclerosis, multiple stenosis, more tortuosity of large arteries, etc. There was also more post-thrombectomy intracranial hemorrhage in older group (10.7% vs. 33%) of this study population. Post-stroke dementias, deterioration of multiple organs or muscle atrophy are another important factor of poor functional outcome at long term follows up in older age group. There are several studies that thrombectomy in elderly patients is benefit only when infarct core is small compared with younger ones61518).
Our analysis revealed that successful recanalization (TICI 2b or 3) was achieved after thrombectomy. After recanalization, 43% of the patients experienced a ≥5-point improvement on the NIHSS at discharge, and 51% of patients achieved a good clinical outcome at 3 months. The reason for the small decrease in the NIHSS of 49% in the patients even after successful recanalization seemed to be associated with persistent hemiparesis/hemiplegia due to core infarct in the motor cortex or corticospinal tract, including the internal capsule, thalamus, and pontomedullary system during the initial stage. Two of 10 patients showed a marked decrease in the NIHSS (≥5) following thrombectomy, even 300 min after symptom onset. This result may have been related to good collaterals and the large volume of salvageable penumbra, even after 300 min. The overall mortality rate in this consecutive series was 5.4%; two of 37 patients died within 3 months.
In many studies, the main prognostic factors are early recanalization, good collateral, small core infarct, low NIHSS, young age, and so on6810151820). In this study, higher TICI grades had intimate relation with larger NIHSS gap and good mRS although shorter time to recanalization, lower initial NIHSS, and higher TIMI had relation only with good mRS. Some patients can achieve good mRS even after delayed recanliazation or only medical therapy if the patients have good collateral. Our purpose of mechanical thrombectomy is to recover and prevent deterioration of neurologic function, not to retain neurologic deficit with good collateral flow. So, our opinion about mechanical thrombectomy is to set a goal of complete recanalization even after some delayed time and poor NIHSS if the patients regarded to have salvageable penumbra.
There are several limitations in this study. First of all, this study is not a randomized controlled study and has selection bias. The number of cases is also small and this is single center study. The decision of endovascular therapy or conservative therapy was made by the physician on duty although we have an indication about mechanical thrombectomy. The uses of variable devices, like balloon, size of stent, were decided case by case. And there is multiple clinical and radiologic factors affecting outcome should be considered and duly weighted besides angiographic factors.
References
1. Akins PT, Amar AP, Pakbaz RS, Fields JD. SWIFT Investigators. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol. 2014; 35:524–528. PMID: 24029392.

2. Alajbegovic A, Djelilovic-Vranic J, Nakicevic A, Todorovic L, Tiric-Campara M. Post stroke depression. Med Arch. 2014; 68:47–50. PMID: 24783913.

3. Bae GS, Kwon HJ, Kang CW, Choi SW, Kim SH, Koh HS. Mechanical thrombectomy using a solitaire stent in acute ischemic stroke; initial experience in 40 patients. J Cerebrovasc Endovasc Neurosurg. 2012; 14:164–169. PMID: 23210042.

4. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20. PMID: 25517348.
5. Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013; 368:893–903. PMID: 23390923.

6. Castonguay AC, Zaidat OO, Novakovic R, Nguyen TN, Taqi MA, Gupta R, et al. Influence of age on clinical and revascularization outcomes in the North American Solitaire Stent-Retriever Acute Stroke Registry. Stroke. 2014; 45:3631–3636. PMID: 25358699.

7. Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368:904–913. PMID: 23387822.

8. Danière F, Lobotesis K, Machi P, Eker O, Mourand I, Riquelme C, et al. Patient selection for stroke endovascular therapy--DWI-ASPECTS thresholds should vary among age groups : insights from the RECOST study. AJNR Am J Neuroradiol. 2015; 36:32–39. PMID: 25273535.
9. de Weerd L, Luijckx GJ, Groenier KH, van der Meer K. Quality of life of elderly ischaemic stroke patients one year after thrombolytic therapy. A comparison between patients with and without thrombolytic therapy. BMC Neurol. 2012; 12:61. PMID: 22835054.

10. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030. PMID: 25671798.

11. Guo RY, Su L, Liu LA, Wang CX. [Effects of Linggui Bafa on the therapeutic effect and quality of life in patients of post-stroke depression]. Zhongguo Zhen Jiu. 2009; 29:785–790. PMID: 19873912.
12. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003; 34:e109–e137. PMID: 12869717.

13. Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013; 368:914–923. PMID: 23394476.

14. Kim TK, Rhim JK, Lee CJ, Oh SH, Chung BS. The Limitations of thrombectomy with Solitaire™ AB as first-line treatment in acute ischemic stroke : a single center experience. J Cerebrovasc Endovasc Neurosurg. 2012; 14:203–209. PMID: 23210048.

15. Kurre W, Aguilar-Pérez M, Niehaus L, Fischer S, Schmid E, Bäzner H, et al. Predictors of outcome after mechanical thrombectomy for anterior circulation large vessel occlusion in patients aged ≥80 years. Cerebrovasc Dis. 2013; 36:430–436. PMID: 24281266.

16. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke : a metaanalysis. Stroke. 2009; 40:2438–2441. PMID: 19478213.

17. Molina CA, Chamorro A, Rovira À, de Miquel A, Serena J, Roman LS, et al. REVASCAT : a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. 2015; 10:619–626. PMID: 24206399.

18. Raoult H, Eugène F, Ferré JC, Gentric JC, Ronzière T, Stamm A, et al. Prognostic factors for outcomes after mechanical thrombectomy with solitaire stent. J Neuroradiol. 2013; 40:252–259. PMID: 23684343.

19. Röther J, Ford GA, Thijs VN. Thrombolytics in acute ischaemic stroke : historical perspective and future opportunities. Cerebrovasc Dis. 2013; 35:313–319. PMID: 23615379.

20. Saake M, Breuer L, Gölitz P, Köhrmann M, Schwab S, Dörfler A, et al. Clinical/perfusion CT CBV mismatch as prognostic factor in intraarterial thrombectomy in acute anterior circulation stroke. Clin Neurol Neurosurg. 2014; 121:39–45. PMID: 24793473.

21. Seifert M, Ahlbrecht A, Dohmen C, Spuentrup E, Moeller-Hartmann W. Combined interventional stroke therapy using intracranial stent and local intraarterial thrombolysis (LIT). Neuroradiology. 2011; 53:273–282. PMID: 20556600.

22. Shindo A, Kawanishi M, Kawakita K, Okauchi M, Kawai N, Hayashi N, et al. Treatment of acute cerebral artery occlusion using the penumbra system : our early experience. Neurol Med Chir (Tokyo). 2014; 54:441–449. PMID: 24759097.

23. Sugg RM, Noser EA, Shaltoni HM, Gonzales NR, Campbell MS, Weir R, et al. Intra-arterial reteplase compared to urokinase for thrombolytic recanalization in acute ischemic stroke. AJNR Am J Neuroradiol. 2006; 27:769–773. PMID: 16611762.
24. Taussky P, Tawk RG, Daugherty WP, Hanel RA. Medical therapy for ischemic stroke : review of intravenous and intra-arterial treatment options. World Neurosurg. 2011; 76(6 Suppl):S9–S15. PMID: 22182278.
25. von Sarnowski B, Kleist-Welch Guerra W, Kohlmann T, Moock J, Khaw AV, Kessler C, et al. Long-term health-related quality of life after decompressive hemicraniectomy in stroke patients with life-threatening space-occupying brain edema. Clin Neurol Neurosurg. 2012; 114:627–633. PMID: 22236827.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download