Abstract
Objective
Anterior communicating artery (AcomA) aneurysms represent the most common intracranial aneurysms and challenging to treat due to complex vascularity. The purpose of this study was to report our experience of endovascular treatment of AcomA aneurysms.
Methods
Between January 2003 and December 2013, we retrospectively reviewed the medical records of 134 AcomA aneurysm patients available more than 6 months conventional angiographic and clinical follow-up results. We focused on aneurismal or AcomA vascular characters, angiographic and clinical follow-up results, and retreatment.
Results
The rate of ruptured cases was 75.4%, and the small (<10 mm) aneurysms were 96.3%. Based on the subtypes defined by dominance of A1, 79 patients (59%) had contralateral A1 hypoplasia or agenesis. The immediate post-procedural angiography confirmed complete occlusion in 75.4%, partial occlusion in 24.6%. Procedure related complications were observed in 25 (18.6%) patients. Most of the adverse events were asymptomatic. Follow-up conventional angiography at ≥6 months was performed in all patients (mean 16.3 months) and major recanalization was noted in 6.7% and regrowth in one case. The aneurysm size (p=0.016), and initial treatment results (p=0.00) were statistically significant risk factors related to aneurysm recurrence. An overall improvement in mRS was observed during the clinical follow-up period and no rebleeding episode occurred.
Conclusion
This study demonstrated that endovascular treatment is an effective treatment modality for AcomA aneurysms with low morbidity. Patients should take long term clinical and angiographic follow-up in order to assess the recurrence and warrant retreatment, especially ruptured, large, and initially incomplete occluded aneurysms.
The anterior communicating artery (AcomA) is one of the most common sites affected by intracranial aneurysms. AcomA aneurysms are diagnosed in 15% to 30% of patients with a ruptured or unruptured aneurysm11131623). In the past, AcomA aneurysms were treated by clipping due to their complex vascularity, but recent improvements in endovascular techniques and instruments have increased the scope of the endovascular approach to aneurismal treatment. However, popularization of the endovascular approach has been accompanied by increases in complications, recurrences, and the retreatment of coiled aneurysms. Compared to surgical clipping, recurrence of embolized aneurysms is still a concern due to their delayed rebleeding.
The aim of present study was to report the long-term outcomes of AcomA aneurysms treatment endovascularly and to document the incidences of complications, recurrences, and retreatment.
The medical records of patients treated by coil embolization for an intracranial saccular aneurysm between January 2003 and December 2013 were retrospectively reviewed. 878 saccular aneurysm cases were treated by endovascular coiling during the study period, and 266 of the 878 patients harbored 266 AcomA aneurysms (30.3%). Only patients with data available for at least 6 months and that underwent digital subtraction angiography (DSA) during follow-up results were included. Two neurovascular surgeons and two neurointerventionists carefully reviewed these angiographs to reduce inter-observer variability. As a result, 132 patients were excluded due to the use of a different follow-up image modality, a non-saccular aneurismal character, or other medical adverse events, such as, vasospasm, lung or cardiac problems. Accordingly, 134 patients (134 AcomA aneurysms) constituted the study cohort.
Patient demographics, aneurysm characteristics, treatment techniques, and angiographic and clinical outcomes were reviewed with a focus on angiographic results, recurrence rate, and retreatment.
Two vascular neurosurgeons and two interventional neuroradiologists evaluated all 134 patients for endovascular treatment. Informed consent for all procedures was obtained from patients or next of kin. The majority of the aneurysms (97%) were coiled under local anesthesia with light sedation. The right femoral artery was accessed using a 6-Fr 80 cm long Shuttle sheath (Cook, Bloomington, IN, USA). A 6-Fr guiding catheter (Envoy; Cordis, Miami Lakes, FL, USA) was then placed in the distal internal carotid artery (ICA) as far as possible. For AcomA aneurysms, proximal back support is immensely important because of their distal location, and thus, a coaxial guiding technique was used in all cases. The ICA ipsilateral to dominant A1 (precommunicating segment of anterior cerebral artery) was catheterized. In cases where A1s were codominant, the A1 with the straightest course to the aneurysm was chosen. After attaining access to the femoral artery, a bolus of 3000 IU heparin was administered intravenously at the beginning of the procedure in cases of non-hemorrhage. However, in cases of aneurysm rupture, heparin was administered after microcatheter selection of an aneurismal sac. Regardless of rupture, an additional 1000 IU bolus of heparin was administered hourly to maintain an activated clotting time (ACT) of ≥250 seconds. All sheaths, guide catheters, and microcatheters were continually flushed with heparinized saline (at a concentration of 1000 IU of heparin per 1000 mL of saline). In non-hemorrhagic cases, dual antiplatelet premedication, consisting of 75 mg clopidogrel and 100 mg aspirin, was administered for 5 days. Postoperatively, dual antiplatelet therapy of clopidogrel and aspirin was maintained for at least 3 months in all cases.
Endovascular treatment consisted of simple coiling (SC), the two catheter technique (TC), balloon-assisted coiling (BAC), or stent-assisted coiling (SAC). Simple coiling, using a single microcatheter, was chosen during the early period, and tailored microcatheter steam shaping was necessary in most cases. However, due to the complex vascularity of the AcomA, the two catheter technique has been adopted in the majority of recent cases. When the two catheter technique could not be applied or failed, adjunctive techniques (balloon-assisted or stent-assisted coiling) were used to achieve satisfactory results. Theoretically, the two catheter technique improves packing density and ensures the coil mass does not protrude into the parent artery. Coils were inserted within aneurysms as densely as possible, or until another coil could not be inserted, without compromising the parent artery. Neck remodeling instruments, such as a balloon or stent, were used when the two catheter technique failed, meaning coil protrusion with flow compromise or unstable shape of coils.
After each procedure, multiple angiographic projections were obtained to assess results, and if there were no complications on final angiography performed 30 minutes after coiling, we completed the whole procedure. Non-enhanced brain computed tomography (CT) was performed immediately after procedures to check for possible complications.
All patients with a subarachnoid hemorrhage (SAH) were initially evaluated according to the Hunt-Hess classification. Grades 0-3 were considered to indicate a good clinical preoperative status and grades 4 and 5 to indicate a poor status. Patients without a history of SAH were considered to have a grade 0 status. Aneurysms were categorized as small (<10 mm) or large (10-24 mm) based on aneurysm maximum length as determined by angiographic analysis. A wide neck was defined as one with a diameter ≥4 mm. Dome to neck ratio was categorized into unfavorable (≤1.5) or favorable (>1.5). Aneurysms with a dome to neck ratio of ≤1.5 or a neck width of ≥4 mm were defined as 'unfavorable neck'. Postoperative and follow-up clinical outcomes were assessed using a modified Rankin Scale (mRS) at discharge and during follow-up. Procedural related permanent morbidity was defined as a change in the mRS associated with intervention. Initial angiographic results were classified using the Raymond classification as complete occlusion, residual neck, or residual aneurysm22). Recurrence of coiled aneurysms was classified as; stable occlusion (SO, defined as no interval change or further obliteration versus the initial post embolization angiogram), major recanalization (MAR, defined as a recanalized volume of ≥20% of the initial aneurismal volume), minor recanalization (MIR, defined as a recanalized volume of <20%), regrowth (RG, defined as the new appearance of aneurismal dilatation or a daughter sac). Using an individual approach, MAR or RG cases were considered candidates for retreatment.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS version 19.0, SPSS Inc., Chicago, IL, USA). Ordinal logistic regression models and simple logistic regression models were used to calculate odds ratios (ORs), corresponding 95% confidence intervals (CIs), and p-values. Statistical significance was accepted for p values of <0.05.
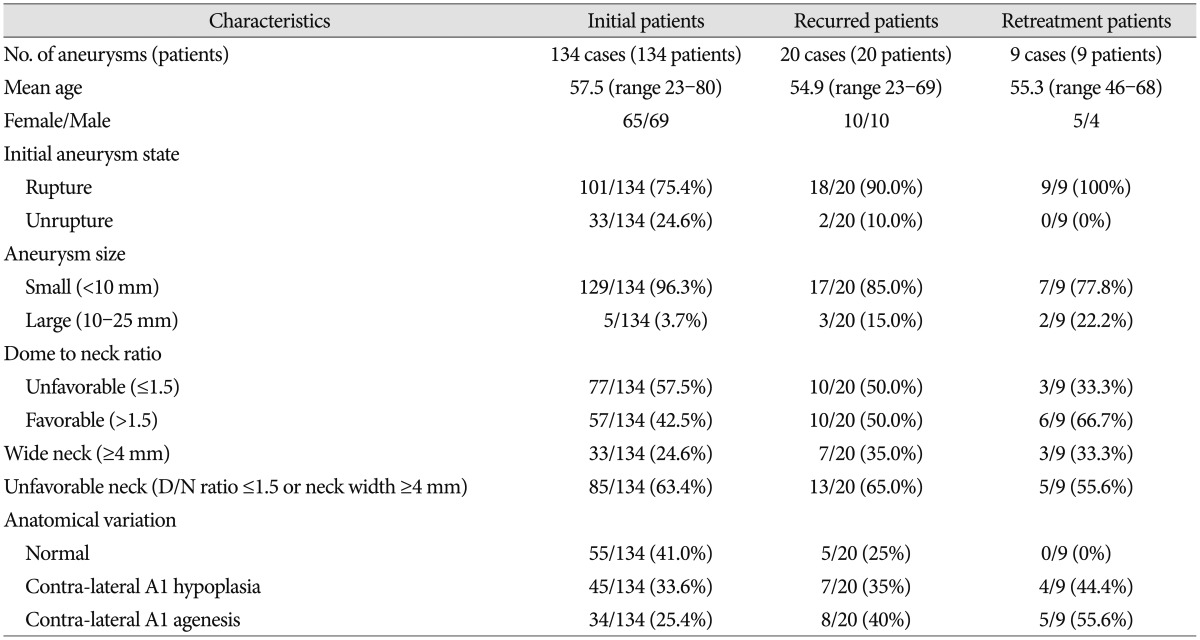
The records of 134 patients (134 AcomA aneurysms) with more than 6 months of angiographic (DSA) and clinical follow-up were analyzed. There were 65 females and 69 males (51.5%) ranging in age from 23 to 80 years (mean 57.4 years). 101 cases (75.4%) presented with SAH and 33 cases (24.6%) were unruptured. 129 aneurysms (96.3%) were small (<10 mm) and 5 were large (10-24 mm). Based on subtypes defined by the angiographic dominance of A1, there were 45 patients (33.6%) with contralateral A1 hypoplasia and 34 patients (25.4%) with contralateral A1 agenesis (Fig. 1). Aneurysms had a wide neck (≥4 mm) in 33 patients (24.6%) and an unfavorable neck in 85 patients (63.4%). Patient demographics are presented in Table 1.
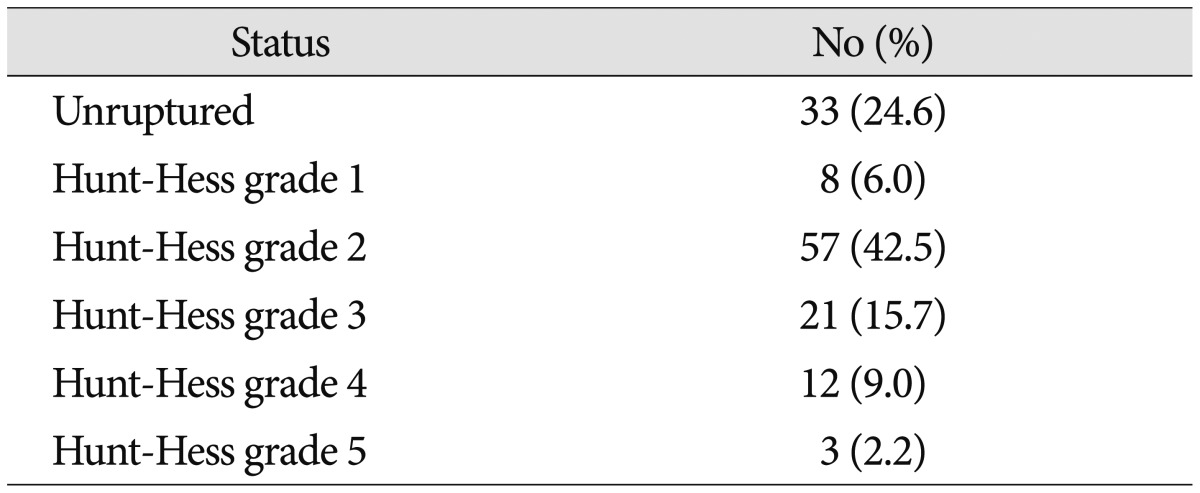
119 patients (88.8%) had a relative good pre-procedural condition (Hunt-Hess grade 0-3). Hunt-Hess grades in ruptured cases were; grade 1 in 8 patients (6.0%), grade 2 in 57 patients (42.5%), and grade 3 in 21 patients (15.7%). 15 patients (11.2%) had a poor pre-procedural condition (Hunt-Hess grades 4-5). Preprocedural clinical statuses are summarized in Table 2.
The coiling techniques and respective patient numbers were as follows : SC in 47 patients (35.1%), TC in 42 patients (31.3%), BAC in 26 patients (19.4%), and SAC in 19 patients (14.2%).
After initial endovascular treatment, complete occlusion was observed in 101 cases (75.4%), a remnant neck in 20 (14.9%), and a remnant aneurysm in 13 (9.7%).
Procedure-related complications, such as, aneurysm rupture or flow compromise (acute thromboembolism, coil migration, or protrusion) were encountered in 25 (18.6%) patients. Aneurismal rupture during treatment occurred for three ruptured aneurysms (2.2%), although these resulted in worsening of patients' initial clinical conditions, they did not appear to have any deleterious effect at discharge. Flow compromise of the distal anterior cerebral artery (ACA) occurred in 22 (16.4%) patients (coil protrusion in 10 and acute thromboembolism in 12). Most cases of coil protrusion with distal flow compromise were successfully managed by stent deployment or balloon angioplasty. Only two cases needed additional thrombolytic agent (abciximab). There were 12 (8.9%) acute thromboembolic episodes. Eight resolved completely on chemical thrombolysis using abciximab, and the other four achieved near complete successful angiographic outcomes [a thrombolysis in cerebral infarction (TICI) score of IIb10)]. The majority of events were asymptomatic, but permanent neurologic deficits due to distal ACA territory infarction (mild lower motor weakness) were noted in two ruptured aneurysm cases (mRS 1). No permanent neurologic deficit was noted among unruptured complicated cases, and no rebleeding episode occurred during clinical follow-up in all patients.
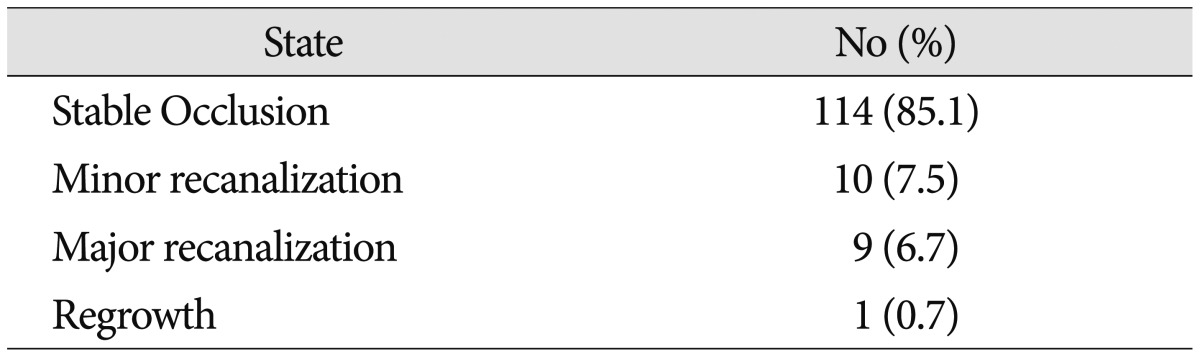
Follow-up conventional angiography at >6 months post-procedurally was performed in all 134 patients. The mean time to follow-up DSA was 16.3 months (range 6 to 73 months). The mean clinical follow-up duration was 49.7 months (range 6 to 139 months). Follow-up DSA revealed stable occlusion (SC) in 114 cases (85.1%) and recurrence in 20 cases (14.9%). There were 10 (7.5%) minor recanalizations (MIR), 9 (6.7%) major recanalizations (MAR), and 1 (0.7%) case of regrowth (RG) (Table 3).
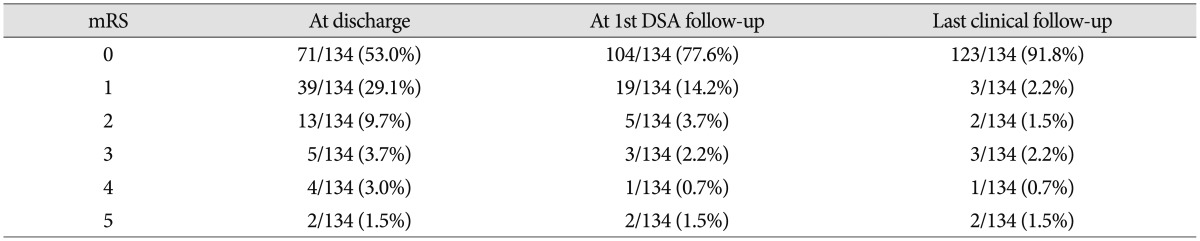
At the time of discharge after initial treatment, 71 (53.0%) patients were of mRS 0, 39 (29.1%) were of mRS 1, 13 (9.7%) were of mRS 2, 5 (3.7%) were of mRS 3, 4 (3.0%) were of mRS 4, and 2 (1.5%) were of mRS 5. At the time of last clinical follow-up, 123 (91.8%) patients were of mRS 0, 3 (2.2%) were of mRS 1, 2 (1.5%) were of mRS 2, 3 (2.2%) were of mRS 3, 1 (0.7%) were of mRS 4, and 2 (1.5%) were of mRS 5. No procedure-related morbidity or mortality occurred among the 33 unruptured cases. An overall improvement in mRS was observed during the clinical follow-up period; changes in mRS scores are summarized in Table 4.
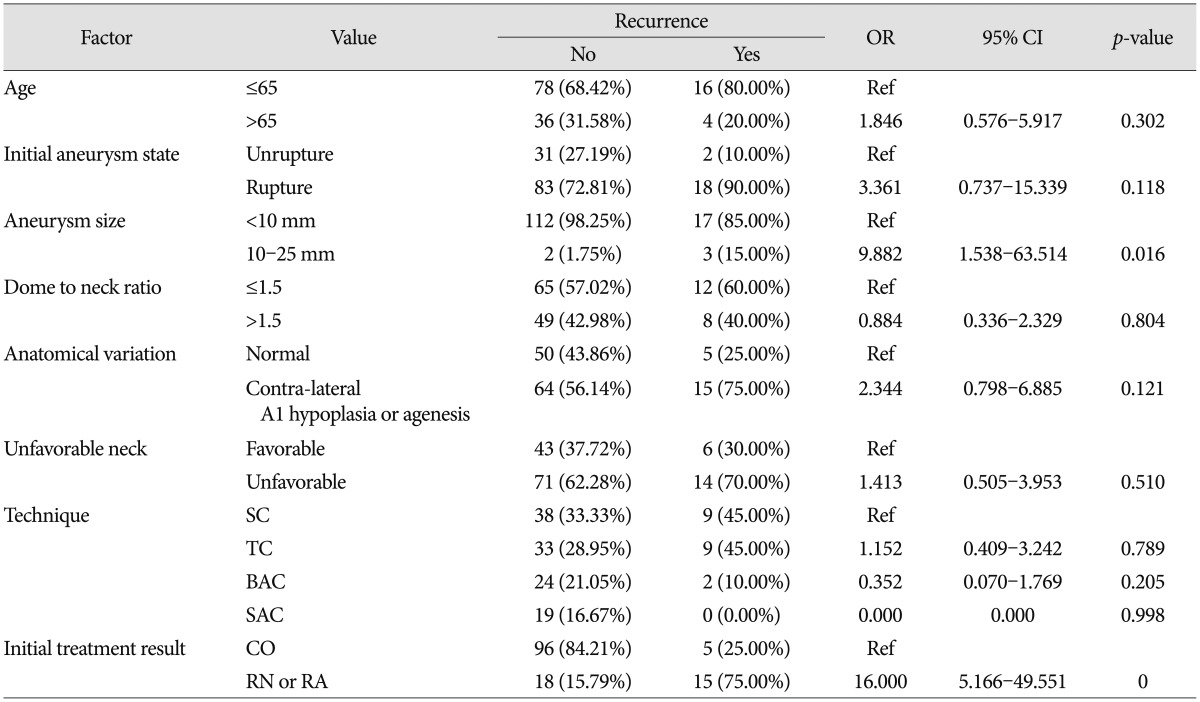
The subgroup analysis of recurred AcomA aneurysms (20 cases) showed a mean age of 54.9 years (range 23-69), 10 (50.0%) female patients, and 18 (90.0%) ruptured aneurysms. 17 (85.0%) patients had an aneurysm size of <10 mm, and 3 (15.0%) patients had an aneurysm size of 10-24 mm. Thirteen (65.0%) cases had an unfavorable aneurysm neck. Seven (35%) cases had contra-lateral A1 hypoplasia and eight (40.0%) contra-lateral A1 agenesis. Simple logistic regression analysis showed that aneurysm size and initial treatment results were significant associated with aneurysm recurrence (Table 5).
Nine (9/134, 6.7%) recurred AcomA aneurysms cases were retreated. Eight involved major recanalization and one regrowth. All (9/9, 100%) cases were ruptured aneurysms. Seven (77.8%) had an aneurysm size of <10 mm and 2 (22.2%) had an aneurysm size of 10-24 mm. Five (5/9, 55.6%) cases had an unfavorable neck. Successful retreatment was achieved in 6 cases (6/9, 66.7%). Three cases failed due to acute angulation of the parent artery or coil migration and are currently awaiting another DSA follow-up. The techniques used were SC in 1 case, TC in 3 cases, BAC in 4, and SAC in 1 case. Mean time from initial treatment to retreatment was 24.2 months (range 8-46 months). There was no procedure-related complication. Fig. 2 shows retreatment of a regrowthed coiled aneurysm.
The anterior communicating artery (AcomA) is frequent site for intracranial aneurysms and accounts for 15% to 30% of ruptured or unruptured aneurysms69111623). Because of the sophisticated vascularity, numerous nearby basal perforators, and anatomical variations of the AcomA, most neurosurgeons have used to prefer clipping AcomA aneurysms. However, depending on direction of the aneurysm sac, the course of the ipsilateral A1, and variations of the A1 artery, surgical clipping can be difficult or complicated in some restricted cases, such as, when the aneurysm has an interhemispheric fissure location. To achieve complete clipping of AcomA aneurysms, while preserving basal perforators, the AcomA and pericallosal arteries could be problematic. On the other hand, endovascular treatment has some advantages in such situations. Advances in endovascular techniques and devices have resulted in the rapid evolution of the endovascular treatment of intracranial aneurysms and it has now become an efficient treatment modality comparable to surgical clipping71112131418). Li et al. reported in a meta-analysis that coiling yields better outcomes than surgical clipping, though the benefit is greater in those with a good preoperative grade12).
However, endovascular treatment of AcomA aneurysms also has some disadvantages as compared with the endovascular treatment of aneurysms in other locations. AcomA aneurysms are difficult to treat when their size is too small, complex aneurysm with incorporation of adjacent arteries, or tortuous parent vascular angulation6). A small number of studies have been conducted on outcomes and complications for the endovascular treatment of AcomA aneurysms. The most recent meta-analysis (14 studies, consisting of 1552 cases) on AcomA coiling was conducted by Fang et al.5). In this study, it was demonstrated that endovascular therapy for AcomA aneurysms is associated with a high rate of complete angiographic occlusion (88%) but not negligible procedure-related morbidity (6%) and mortality (3%). Complete occlusion is a necessary goal of endovascular treatment, and in the present study, complete occlusion percentage achieved after initial treatment was 75.4%, which is in close agreement with several reports that quoted rates of complete occlusion from 35.9% to 88% for AcomA aneurysms5671419). Our procedure related complication rate was 16.4%, which is also similar to previous studies, but no rebleeding was encountered during follow-up period, the morbidity rate was 1.5%, and procedure related mortality rate was 0%. Accordingly, our procedural complication rates appear to be better than those reported in other studies56814). In our opinion, satisfactory occlusion effectively protected against the risk of rebleeding, and thus, we emphasize the importance of achieving complete occlusion at initial treatment. Fortunately, no rebleeding episode occurred during clinical follow-up period considering the lower initial complete occlusion rate (75.4%), although this relatively lower rate may have been due to strict and multiple cross checking of post-procedural angiographies.
Currently the imaging modalities used for follow-up after initial treatment to determine recurrence of coiled aneurysms and find de novo aneurysms are magnetic resonance angiography (MRA), computed tomography angiography (CTA), and DSA. Given advancements in MRA resolution and image technique, it appears to be a promising technique for reliably imaging coiled aneurysm recurrence. However, DSA remains the most accurate tool for evaluating recurrence, because it could compare angioarchitectures of the aneurysm through best exactly the same projection. Thus, the use of DSA as a follow-up image modality could provide a means to reduce interpretative errors regarding recurrence. Therefore, DSA is considered mandatory when considering retreatment. In the present study, we analyzed the data of patients that underwent DSA at >6 months post-procedurally, and the recurrence rate in this population was 14.9%. Two neurointerventionists and two neurosurgeons cross-checked DSA follow-up results regarding recurrence and decisions to retreat, and our recurrence rate result is similar to those of several studies on the recurrence rate of various aneurysms (range 6.1-33.6%)291821). However, we believe our recurrence rate (14.9%) was somewhat overestimated in comparison because follow-up was conducted by DSA. The majority of recurrences occurred during the first year, but some occurred at up to 5 years post-treatment. Therefore, long-term follow-up is mandatory, especially in ruptured cases.
Several factors that influence aneurysm recurrence have been identified, such as, initial angiographic result, packing density ratio, aneurysm anatomy, and aneurysmal nature and size24911182021). In the present study, the significant risk factors of recurrence identified were a large aneurysm (p=0.016) and an initial incomplete treatment result (p=0), which are generally recognized as predictors of recurrences for all aneurysm locations. Furthermore, an initially ruptured aneurysm (OR=3.36) and contralateral A1 variation such as hypoplasia or ageneis (OR=2.33), tended to be associated with recurrence. In the present study, the overall retreatment rate was 6.7% (9/134) at a mean 24.2 months after initial treatment, which agrees with literature values of 4.7-20.8%1234789192021). The majority of retreatments were performed within 2 years after initial treatment, although three retreatment cases failed due to acute angulation of the parent artery or coil migration, most patients were retreated successfully without complication.
In general, the diameters of both A1 segments are similar, however, some have reports about high proportion of AcomA variation15172425). A true hypoplastic A1 or A1 segment measuring <1.5 mm in diameter is only present in 10% of cases15). The larger the degree of asymmetry between A1 segments, the greater the hemodynamic stress loaded on the dominant A1 artery to compensate distal ACA flow. Theoretically, this asymmetry could facilitate de novo aneurysm formation. High prevalences of A1 hypoplasia among AcomA aneurysms patients have been reported (up to ~80%)2425), and our study supports these findings (59%).
The limitations of this study include a retrospective design, patient-selection bias, a limited number of cases in one institution, and the relatively short angiographic follow-up period. Nevertheless, our findings suggest endovascular treatment for AcomA aneurysms is feasible, safe, and effective. Compensatory endovascular and microsurgical team approaches are needed to optimally treat AcomA aneurysms.
This study confirms that endovascular treatment is an effective treatment modality for AcomA aneurysms. The hemody-namics and anatomic features of the AcomA should be considered before selecting endovascular treatment. After initial treatment, patients should comply with long-term clinical and angiographic follow-up for the assessment of recurrence and consideration of possible retreatment, especially when aneurysms are ruptured, large, or initially incomplete occluded.
References
1. Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms : outcomes and incidence of late rebleeding. J Neurosurg. 1999; 90:656–663. PMID: 10193610.

2. Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke. 2007; 38:1538–1544. PMID: 17395870.

3. Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999; 212:348–356. PMID: 10429689.

4. Corns R, Zebian B, Tait MJ, Walsh D, Hampton T, Deasy N, et al. Prevalence of recurrence and retreatment of ruptured intracranial aneurysms treated with endovascular coil occlusion. Br J Neurosurg. 2013; 27:30–33. PMID: 22762269.

5. Fang S, Brinjikji W, Murad MH, Kallmes DF, Cloft HJ, Lanzino G. Endovascular treatment of anterior communicating artery aneurysms : a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2014; 35:943–947. PMID: 24287090.

6. Finitsis S, Anxionnat R, Lebedinsky A, Albuquerque PC, Clayton MF, Picard L, et al. Endovascular treatment of ACom intracranial aneurysms. Report on series of 280 patients. Interv Neuroradiol. 2010; 16:7–16. PMID: 20377974.

7. Gallas S, Januel AC, Pasco A, Drouineau J, Gabrillargues J, Gaston A, et al. Long-term follow-up of 1036 cerebral aneurysms treated by bare coils : a multicentric cohort treated between 1998 and 2003. AJNR Am J Neuroradiol. 2009; 30:1986–1992. PMID: 19679641.

8. Gonzalez N, Sedrak M, Martin N, Vinuela F. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke. 2008; 39:2776–2782. PMID: 18617670.

9. Henkes H, Fischer S, Liebig T, Weber W, Reinartz J, Miloslavski E, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms safety and effectiveness aspects. Neurosurgery. 2006; 58:224–232. discussion 224-232PMID: 16462475.

10. Higashida R, Furlan A, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S493–S494. PMID: 14514864.

11. Kwon SC, Kwon OK. Korean Unruptured Cerebral Aneurysm Coiling (KUCAC) Investigators. Endovascular coil embolization of unruptured intracranial aneurysms : a Korean multicenter study. Acta Neurochir (Wien). 2014; 156:847–854. PMID: 24610449.

12. Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, et al. Clipping versus coiling for ruptured intracranial aneurysms : a systematic review and meta-analysis. Stroke. 2013; 44:29–37. PMID: 23238862.

13. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms : a randomised trial. Lancet. 2002; 360:1267–1274. PMID: 12414200.

14. Moret J, Pierot L, Boulin A, Castaings L, Rey A. Endovascular treatment of anterior communicating artery aneurysms using Guglielmi detachable coils. Neuroradiology. 1996; 38:800–805. PMID: 8957810.

15. Nathal E, Yasui N, Sampei T, Suzuki A. Intraoperative anatomical studies in patients with aneurysms of the anterior communicating artery complex. J Neurosurg. 1992; 76:629–634. PMID: 1545257.

16. Ogungbo B, Gregson BA, Blackburn A, Mendelow AD. Newcastle Subarachnoid Study Group. Trends over time in the management of subarachnoid haemorrhage in newcastle : review of 1609 patients. Br J Neurosurg. 2001; 15:388–395. PMID: 11708541.

17. Perlmutter D, Rhoton AL Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976; 45:259–272. PMID: 948013.

18. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID: 12775880.

19. Raymond J, Kotowski M, Darsaut TE, Molyneux AJ, Kerr RS. Ruptured aneurysms and the International Subarachnoid Aneurysm Trial(ISAT): What is known and what remains to be questioned. Neurochirurgie. 2012; 58:103–114. PMID: 22481029.

20. Renowden SA, Koumellis P, Benes V, Mukonoweshuro W, Molyneux AJ, McConachie NS. Retreatment of previously embolized cerebral aneurysms : the risk of further coil embolization does not negate the advantage of the initial embolization. AJNR Am J Neuroradiol. 2008; 29:1401–1404. PMID: 18436614.

21. Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. 2007; 28:1755–1761. PMID: 17885238.

22. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001; 32:1998–2004. PMID: 11546888.

23. Sim JH. Intracranial aneurysms in Korea. Neurol Med Chir (Tokyo). 1998; 38(Suppl):118–121. PMID: 10234990.

24. Stehbens WE. Aneurysms and anatomical variation of cerebral arteries. Arch Pathol. 1963; 75:45–64. PMID: 14087271.
25. Wilson G, Riggs HE, Rupp C. The pathologic anatomy of ruptured cerebral aneurysms. J Neurosurg. 1954; 11:128–134. PMID: 13152563.

Fig. 1
Images of anterior communicating artery aneurysms with contralateral A1 hypoplasia (A-D) and agenesis (E-H). A and B : Right and left internal carotid artery angiographies demonstrate left anterior communicating artery aneurysm with right A1 hypoplasia (black arrow). C : Final image after coiling shows complete occlusion of the aneurysm. D : The 6 months follow-up angiogram confirms stable occlusion of the coiled aneurysm. E and F : Both internal carotid arteries digital subtraction angiographies shows left anterior communicating artery aneurysm with contralateral A1 agenesis. G and H : Angiography immediately after coiling and 2 years follow-up image confirms complete and stable occlusion of the aneurysm sac.
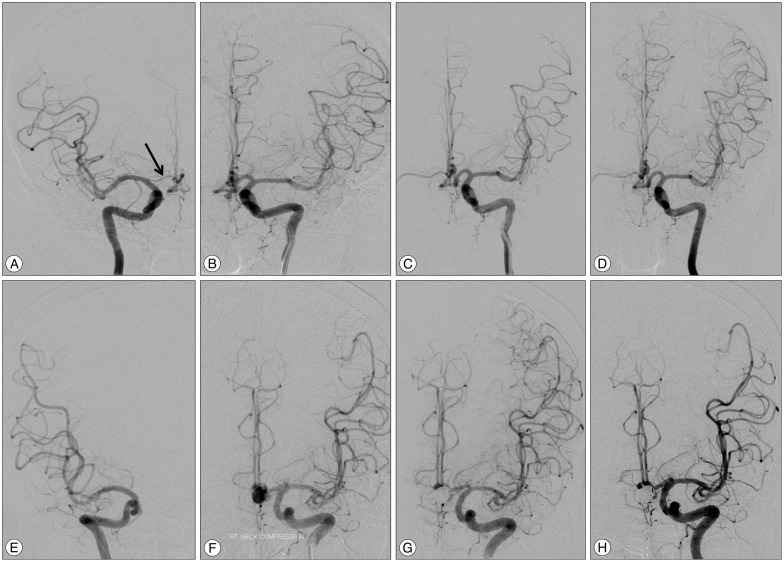
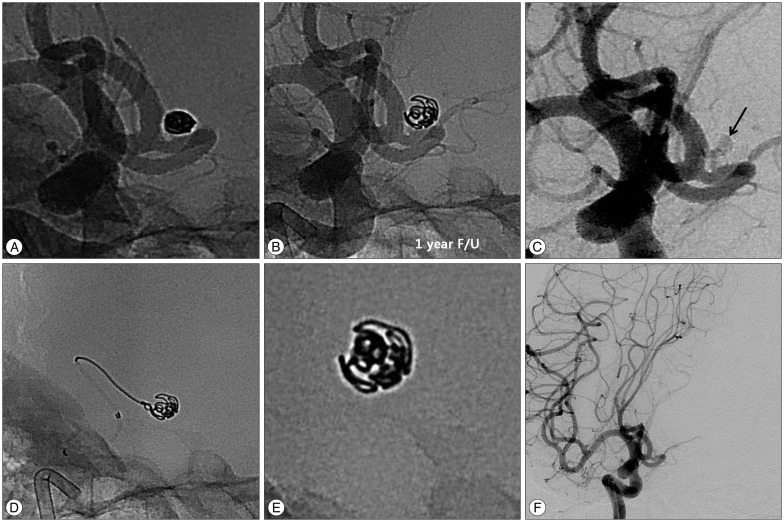
Fig. 2
Retreatment of a 51-years-old female patient with recurred aneurysm. A : Initial unsubtracted image of conventional angiography shows complete occlusion of an aneurysm sac. B : Unsubtracted image of 1 year follow-up demonstrates enlarged and loosened coil mass. C : Subtracted image of previously coiled aneurysm reveals contrast filling of the aneurysm sac (black arrow, regrowth). D : Retreatment of recurred aneurysm using simple coiling technique. E : X-ray image of retreated coil mass. F : Final angiography after retreatment demonstrates complete occlusion of the recurred aneurysm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download