Abstract
The prophylactic use of phenytoin during and after brain surgery and cranial irradiation is a common measure in brain tumor therapy. Phenytoin has been associated with variety of adverse skin reactions including urticaria, erythroderma, erythema multiforme (EM), Stevens-Johnson syndrome, and toxic epidermal necrolysis. EM associated with phenytoin and cranial radiation therapy (EMPACT) is a rare specific entity among patients with brain tumors receiving radiation therapy while on prophylactic anti-convulsive therapy. Herein we report a 41-year-old female patient with left temporal glial tumor who underwent surgery and then received whole brain radiation therapy and chemotherapy. After 24 days of continous prophylactic phenytoin therapy the patient developed minor skin reactions and 2 days later the patient returned with generalized erythamatous and itchy maculopapuler rash involving neck, chest, face, trunk, extremities. There was significant periorbital and perioral edema. Painful mucosal lesions consisting of oral and platal erosions also occurred and prevented oral intake significantly. Phenytoin was discontinued gradually. Systemic admistration of corticosteroids combined with topical usage of steroids for oral lesions resulted in complete resolution of eruptions in 3 weeks. All cutaneous lesions in patients with phenytoin usage with the radiotherapy must be evoluated with suspicion for EM.
Treatment of intracranial malignancies includes prophylactic anti-convulsant and steroid medication in addition to a combination of surgery, radiation therapy and chemotherapy. Phenytoin as a anti-convulsant therapy has been known for variety of adverse skin reactions including urticaria, erythroderma, erythema multiforme (EM), Stevens-Johnson syndrome, and toxic epidermal necrolysis for a long time246101113). Erythema multiforme (EM) is a skin reaction that ranges from self-limited cutaneous eruption to a progressive mucocutaneous disease145891315). EM typically presents with self-limited targetoid lesions on the extensor surfaces of extremities and is often associated with underlying infection (commonly herpes simplex) or a hypersensitivity reaction to a variety of medications like sulfonamides, penicillines, quinolones and anti-convulsants124611).
Hypersensitivity reaction mechanism caused by anti-convulsant drugs is not clear however, it has been proposed that toxic metabolite of aromatic anti-convulsants could bind the cellular macromolecules causing a secondary immunological response3). In patients receiving phenytoin as a prophylactic anti-convulsant who have also treated with radiotherapy, anti-eppileptics must be administered with caution, and all cutaneous reactions developing subsequently within the radiation site must be promptly evaluated with a high index of suspicion for erythema multiforme.
Herein we report the case of a patient who developed EM after administration of cranial irradiation and phenytoin treatment.
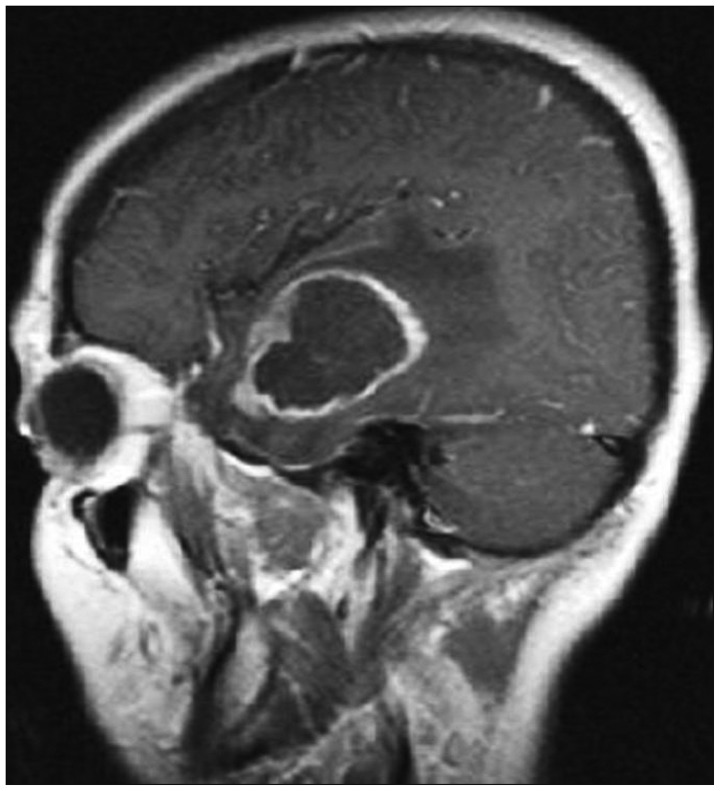
A 41-year-old female patient with left temporal lobe WHO grade IV glioblastome multiforme underwent surgery on the first day after admitting to our hospital (Fig. 1). The patient was given 300 mg/day of phenytoin and 16 mg/day dexamethasone before the surgery. Upon initial diagnosis of glioblastoma multiforme (GBM) after maximal surgical resection the patient underwent radiotherapy, and concomitant and adjuvant chemotherapy with temozolomide. She continued taking phenythoin and tapering dosage of dexamethasone. She subsequently underwent whole brain radiation therapy and was given a total dose of 6000 cGy over 5 days per week in doses of 2.0 Gy fractions spaced over 6 weeks. The patient underwent chemotherapy with temozolomide 130 mg/day for 42 days. After 1 month without chemotherapy she was given 6 cycles of 300 mg/day temozolomide for 5 days.
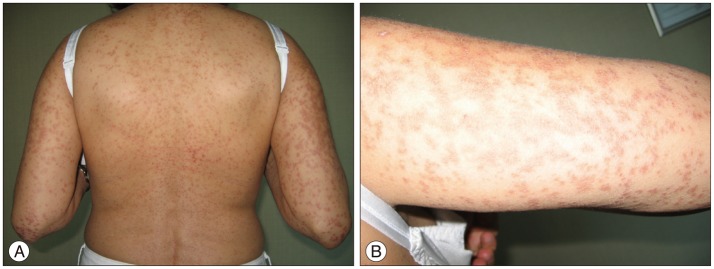
After completing radiation therapy and tapering of dexamethasone on the 24th day of continous prophylactic phenytoin therapy the patient developed minor skin reactions on the scalp within the radiation field especially on left temporal side. The maculopappular eruption generalized within a few days to involve the neck, face, trunk and extremities (Fig. 2). Edema on the lips, mucosal lesions consisting of oral erosions, conjunctival suffusion and periorbital edema also evolved. Laboratory serology included a normal blood cell differential with 12% monocytes, 1.9% eosinophils. Transaminase levels were midly elevated-aspartate aminotransferase 49 U/L and alanine aminotransferase 57 U/L. Anti-nuclear antibody (ANA), anti-double stranded DNA (Anti-dsDNA), anti-extractable nuclear antigen (Anti-ENA), anti-neutrophil cytoplasmic antibody (ANCA) were negative and serum immunoglobulin levels were normal. The was no clinical and/or laboratory evidence of a infection or an autoimmune disease.
Dermatological consultation was sought and a diagnosis of phenytoin induced EM was considered. Phenytoin was discontinued and patient received intravenous corticosteroid that was tapered over 4 days. The patient was also given topical corticosteroid for oral ulcerations. On the 6th day of discontinuation of phenytoin patient had temporal lobe seizure and levetiracetam 2000 mg/day and carbamezapine 400 mg/day was initiated for seizure prophylaxis. Over the following 1 week resolution of the mucocutaneous lesions occured and the patient completely recovered within 3 weeks after stopping phenytoin.
EM is a mucocutaneous reaction associated with several precipitating factors that include infections and various drugs such as sulfonamides, penicilinle, anti-convulsants, allopurinol and anti-inflammotory drugs1347810). EM typically presents with self-limited targetoid lesions on the extensor surfaces of extremities but skin lesions range from skin rashes to a progressive mucocutaneous disease145891315).
Phenytoin is commonly prescribed as a prophylactic anti-convulsant in patients with intracranial malignancies. Phenytoin induced skin reactions ranging from urticaria to toxic epidermal necrolysis has been reported several times12346891112131415). Skin reactions associated with anti-convulsant therapy mainly occur during the first few days to 8 weeks of anti-convulsant drug administration81011). As in our case skin lesions occured 24 days after continous prophylactic phenytoin therapy.
A generalized hypersensitivity has also been described in approximately 10% of patients receiving phenytoin that consists of fever, arthralgias, peripheral eosinophilia, generalized lymphadenopathy and hepatosplenomegaly1378). This hypersensitivity reaction frequently associated with aromatic anti-convulsants such as phenytoin, phenobarbital and carbamezapine. Anti-convulsants are converted to reactive metabolites and induce cytochrome P450 3A and produce oxidative reactive intermediates that may be implicated in hypersensitivity reactions and then these metabolites excreted from kidney via another hepatic enzyme epoxide hydrolase23589101113). Imbalance between the formation of metabolites and enzymatic detoxification leads to accumulation of metabolites causes them to bind with celluler macromoleculles resulting in a toxic role in type 4 hypersensitivity reactions135810). Baba et al.3) suggested that hypersensitivity reactions developed after craniotomy and surgical trauma could have been involved in decreasing activity of hepatic enzymes due to exposure to anaesthetic agents3). Thus this is a clinical phenomenon that occurs with unusual frequency in patients with brain tumor who undergo radiation therapy while taking phenytoin or other anticonvulsants such as phenobarbital and carbamazepine8). Both carbamazepine and barbiturates have shown cross-sensitivity with phenytoin
Recently management of glioblastoma multiforme progressively changed to maximal surgical resection with postoperative radiotherapy and concurrent chemotherapy. In our case after maximal surgical resection the patient underwent radiotherapy, and concomitant and adjuvant chemotherapy with temozolomide. Several reports in literature suggest the existance of a phenomenon in which EM, Stevens-Johnson syndrome or toxic epidermal necrolysis may ocur in patients with brain tumors treated with radiation therapy and anti-convulsant drugs and it was concluded that radiation therapy was responsible because reinstitution of the drugs did not result in recurrence of the reaction. As in our case the clinical picture differed from the classic form of EM in that the erythema began on the scalp and spread to the extremities, progressing to extensive bullous formation16810). As a well recognized phenomenon cutaneous reactions with radiation therapy is usually dose dependent and consists of erythema limited to the radiation field14610). Irradiation can enhance a primary antibody response and impairs T suppressor cells and radiation therapy might promote the development of a hypersensitivity reaction to phenytoin that may be more evident patients receive a tapering dosage of steroids8).
Ahmed et al.1) recently used definition EMPACT (E : erythema M : multiforme associated with P : phenytoin and C : cranial radiation T : therapy) to best describe this disorder. Ahmed et al. reviewed 24 patients which had taken phenytoin for variable time periods (mean 40 days) the lesions developed within the port site during the radiation treatments or soon after its completion. In literature many cases, as in our case, the skin rashes and eruptions first ocur in the irradiation area which may resulted in misdiagnosis as a normal radiation reaction and this may cause delaying the diagnosis of EM lesions and more severe syndromes246810). In literature as in our case not only skin eruptions but EM lesions on the irradiation area with pheytoin treatment was described as diagnostic criteria for EMPACT1). It is also reported that the incidence of this syndrome is much more frquent in cases where phenytoin was administered less than 2 months prior to start of radiotherapy, compared to cases where treatment commenced more then 2 months before irradiation1013).
The optimal treatment for EM is not well defined. Phenytoin should be discontinued immediatelly and high dose intravenous steroid application resolves dermal lesions. For the oral mucosal lesions topical steroid is necessarry8101113). If the lesions are more severe like Stevens-Johnson syndrome or toxic epidermal necrolysis sympthomatic treatment should be applied similiar to that used for burns. Metabolic and electrolyte imbalance should be corrected by replacement of intravenous fluids.
The prophylactic use of anti-convulsants after brain surgery or during cranial irradiation is very common. In patients receiving phenytoin as a prophylactic anti-convulsant who have also treated with radiotherapy EM is a relatively rare adverse situation. The physician must be aware of the anti-convulsant hypersensitivity syndrome when combined with radiotherapy and all cutaneous reactions developing subsequently within the radiation field must be evaluated with the suspicion of EM. In patients with disseminated rashes phenytoin administration should be discontinued immediately and intravenous steroid treatment should be given and if necessary sympthomatic and supportive treatment should be started similiar to that given to patients with burns.
References
1. Ahmed I, Reichenberg J, Lucas A, Shehan JM. Erythema multiforme associated with phenytoin and cranial radiation therapy : a report of three patients and review of the literature. Int J Dermatol. 2004; 43:67–73. PMID: 14693027.

2. Arif H, Buchsbaum R, Weintraub D, Koyfman S, Salas-Humara C, Bazil CW, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007; 68:1701–1709.

3. Baba M, Karakaş M, Aksungur VL, Homan S, Yücel A, Acar MA, et al. The anticonvulsant hypersensitivity syndrome. J Eur Acad Dermatol Venereol. 2003; 17:399–401. PMID: 12834448.

4. Barbosa LA, Teixeira CR. Erythema multiforme associated with prophylactic use of phenytoin during cranial radiation therapy. Am J Health Syst Pharm. 2008; 65:1048–1050. PMID: 18499877.

5. Lin MS, Dai YS, Pwu RF, Chen YH, Chang NC. Risk estimates for drugs suspected of being associated with Stevens-Johnson syndrome and toxic epidermal necrolysis : a case-control study. Intern Med J. 2005; 35:188–190. PMID: 15737140.

6. Mamon HJ, Wen PY, Burns AC, Loeffler JS. Allergic skin reactions to anticonvulsant medications in patients receiving cranial radiation therapy. Epilepsia. 1999; 40:341–344.

7. Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007; 48:1015–1018. PMID: 17509004.

8. Micali G, Linthicum K, Han N, West DP. Increased risk of erythema multiforme major with combination anticonvulsant and radiation therapies. Pharmacotherapy. 1999; 19:223–227. PMID: 10030773.

9. Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005; 64:1134–1138. PMID: 15824335.

10. Oner Dincbas F, Yörük S, Demirkesen C, Uzel O, Koca S. Toxic epidermal necrolysis after cranial radiotherapy and phenytoin treatment. Onkologie. 2004; 27:389–392. PMID: 15347896.

11. Pelekanos J, Camfield P, Camfield C, Gordon K. Allergic rash due to antiepileptic drugs : clinical features and management. Epilepsia. 1991; 32:554–559. PMID: 1831121.

12. Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995; 333:1600–1607. PMID: 7477195.

13. Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy : a case-control study. Lancet. 1999; 353:2190–2194. PMID: 10392983.

14. Welykyj S, Gradini R, Nakao J, Massa M. Carbamazepine-induced eruption histologically mimicking mycosis fungoides. J Cutan Pathol. 1990; 17:111–116. PMID: 2140116.

15. Wöhrl S, Loewe R, Pickl WF, Stingl G, Wagner SN. EMPACT syndrome. J Dtsch Dermatol Ges. 2005; 3:39–43. PMID: 16353748.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download