Abstract
Objective
To investigate the incidence of corpus callosum injury (CCI) in patients with mild traumatic brain injury (TBI) using brain MRI. We also performed a review of the clinical characteristics associated with this injury.
Methods
A total of 356 patients in the study were diagnosed with TBI, with 94 patients classified as having mild TBI. We included patients with mild TBI for further evaluation if they had normal findings via brain computed tomography (CT) scans and also underwent brain MRI in the acute phase following trauma. As assessed by brain MRI, CCI was defined as a high-signal lesion in T2 sagittal images and a corresponding low-signal lesion as determined by axial gradient echo (GRE) imaging. Based on these criteria, we divided patients into two groups for further analysis : Group I (TBI patients with CCI) and Group II (TBI patients without CCI).
Results
A total of 56 patients were enrolled in this study (including 16 patients in Group I and 40 patients in Group II). Analysis of clinical symptoms revealed a significant difference in headache severity between groups. Over 50% of patients in Group I experienced prolonged neurological symptoms including dizziness and gait disturbance and were more common in Group I than Group II (dizziness : 37 and 12% in Groups I and II, respectively; gait disturbance : 12 and 0% in Groups I and II, respectively).
Mild traumatic brain injury (TBI) accounts for over 70% of all reported TBI cases, with an overall proportion of hospitalized patients of approximately 0.1 to 0.3 per 100000 in the population 1141524). This number increases to 0.6 per 100000 when including those who have not been treated at the hospital2). Despite usually favorable outcomes, some patients with mild TBI have prolonged clinical presentations or permanent impairments. However, these conditions do not always correspond to brain abnormalities visible via computed tomography (CT); organic lesions are sometimes not initially detected, without further evaluation, when patients report relatively benign symptoms. Anatomically, the corpus callosum, which links the left and right cerebral hemispheres, is vulnerable to head trauma18172426). In a recent study, the incidence of corpus callosum injury (CCI) in patients with TBI was considerably high141619). We can therefore assume that CCI will be detected in a population of patients with minor head trauma. In this study, we aimed to investigate the incidence of CCI in patients with mild TBI presenting with normal brain CT scan results using brain magnetic resonance imaging (MRI). We also reviewed patient medical records to assess the associated clinical characteristics.
We evaluated the medical records of 356 patients with TBI who were admitted to the neurosurgery department from January 2011 to January 2014. A total of 94 patients were identified with mild cases of TBI that fulfilled the following criteria (as stipulated by the American Congress of Rehabilitation Medicine) : loss of consciousness with a duration less than 30 min, an initial Glasgow Coma Scale (GCS) of 13 to 15, and posttraumatic amnesia lasting 24 h or less. Patients with combined injuries, such as trauma to face, chest, abdomen, or extremities, were excluded. We included patients with normal brain CT scan results and who also underwent brain MRI (using the Philips Achieva 1.5 T system, Philips, Eindhoven, The Netherlands) in the acute phase following trauma (within 2 weeks) as a result of persisting neurological symptoms including uncontrolled headache, dizziness, memory impairment, and gait disturbance. We divided the enrolled patients into two groups : group I (patients with CCI), and group II (patients without CCI). Acute CCI was diagnosed according to the following criteria : patients who had increased signal intensity in regions of the corpus callosum on a T2-weighted sagittal image [repetition time (TR)/echo time (TE) : 3500/100 ms] with decreased signal intensity in corresponding regions as assessed by axial Gradient Echo (GRE, TR/TE : 660/23 ms) imaging. We categorized CCI based on damage to 5 separate regions of the corpus callosum : the anterior third (I), anterior midbody (II), posterior midbody (III), isthmus (IV), and splenium (V), according to "Witelson's classification" (Fig. 1)1323). We then analyzed the incidence, location of injury, and clinical characteristics (including clinical presentations, mode of trauma, treatment duration, and clinical outcomes at the last visit).
Demographics and clinical data were evaluated using the Mann-Whitney U test. The Pearson's chi-square test was used to analyze nominal data, and to ensure that the two groups were comparable. Null hypotheses were rejected if p-values were less than 0.05. Data were analyzed using SPSS 21.0 statistical software (SPSS V21.0K, IBM Inc., Chicago, IL, USA).
The demographic and clinical characteristics of 56 patients are shown in Table 1. Both groups of patients had similar population characteristics with respect to age, gender, brain MRI examination time following trauma, and treatment duration. The clinical symptoms described were headache, dizziness, memory impairment, and gait disturbance. Upon admission, the visual analogue scale (VAS) scores for headache severity were significantly different between groups (with scores of 4.2 for Group I, and 2.7 for Group II; p=0.022). Dizziness and gait disturbances were more prevalent in Group I compared with Group II; however, dizziness was not statistically significant (p=0.912).
Upon evaluating the modes of injury, slipping was the most common cause of head trauma in both groups (50% and 40% of patients for Groups I and II, respectively). Traffic accidents were also noted as a common cause of injury, with 5 patients admitted for this cause in Group I and 13 patients similarly admitted in Group II. Although falling down was rare in Group I, there were no significant differences in modes of trauma between groups.
Sixteen of the 56 patients (29%) we evaluated using brain MRI presented with CCI, and were subsequently classified into Group I. Clinical assessments and MRI findings for these patients are shown in Table 2. The mean age of this group was 51 years (range, 13-77 years) with a predominance of male patients. Among the patients, 4 were diagnosed with skull fractures, and the remaining individuals were diagnosed with cerebral concussions. Upon analysis of the modes of trauma, slipping was a cause for 8 patients, traffic accidents for 5 patients, and assault for 3 patients. In addition to a headache, which was found in all patients, 8 patients (50%) reported other clinical symptoms including dizziness, gait disturbance. A single patient reported having all of these symptoms, with a severe headache. Loss of consciousness was noted in 4 patients. Hospital stays ranged from 2 days to 14 days, and the Glasgow outcome scale score was 5 for all patients, who reported no further complications.
A total of 80 possible CCI regions were evaluated in all patients; CCI was subsequently observed in 19 regions were following brain MRI. Among the damaged regions we identified, 14 (74%) were located in the anterior two-thirds of the corpus callosum. Additionally 3 individuals (patients 2, 8, and 12) had trauma in two regions of the corpus callosum. Damage was identified in the isthmus region (IV) for 1 person (patient 12) and splenium region (V) in 4 individuals (patients 3, 4, 9, and 12).
A 73-year-old man visited the emergency room after traffic a accident reporting headache and dizziness. Neurological evaluation revealed mental alertness without any motor and sensory deficits in any limbs. The patient received an initial GCS score of 15, and had a 5-cm, horizontal laceration to the eyebrow region on his right side. Upon admission, brain CT imaging showed no hemorrhage lesions (Fig. 2A), and he received a diagnosis of cerebral concussion. Three days after the initial trauma, he reported experiencing a persistent, severe headache, with a VAS score of 6, and dizziness. A brain MRI was then performed to evaluate the cause of clinical discomfort. The MRI scan revealed high signal intensity in region III of the corpus callosum on a T2-weighted sagittal image (Fig. 2B). Fortunately, the patient's condition improved within 7 days, and he required no further treatment. A brain CT scan was performed during a follow-up visit 3 months later with, which showed results consistent with initial MRI findings (Fig. 2C). During this visit, his neurological and functional status was normal, and no additional complications related to the TBI were present.
The variable nature of TBI presents numerous problems for medical and psychological assessment, treatment, and outcome predictions21726). However, as a result of spontaneous resolution and improvement of function, unexplained or relatively benign clinical presentations of mild TBI are sometimes neglected or overlooked. Some reports have revealed a high incidence of patients with TBI in which CCI was related to their clinical symptoms; in a recent study, fiber tractography using diffusion tensor MRI was performed to identify changes in the microstructural integrities of patient brains with diffuse axonal injury after trauma3891316).
In the present study, 56 enrolled patients were classified as having mild TBI, with 29% of these patients (n=16) showing MRI signal changes in regions of the corpus callosum without the appearance of abnormal lesions via brain CT. This incidence of 29% is much higher than was initially expected. The corpus callosum, which is largest white matter tract in the brain, is considered to be particularly vulnerable to traumatic head injury due to its unique location and composition12781012). Some authors have reported that focal lesions produced by shearing mechanisms are particularly common in the splenium, and identified long-term diffuse atrophy in the corpus callosum in adult TBI cases via brain MRI135). In 1988, Gentry et al.9) performed a prospective design study of 63 patients with TBI in which the frequency, distribution, and appearance of CCIs were evaluated using brain CT and MRI procedures. The authors of this study found that lesions were present in 31 (49.2%) of all patients; among these patients, 21 cases (67.7%) were non-hemorrhagic, with CCI detected in 33% of these cases with acute TBI9). The location of CCI was classified according to neuroanatomical divisions into 4 segments : the rostrum, genu, body, and splenium. The authors reported that the frequency of CCI progressively increased anteriorly to posteriorly, and injuries to the splenium were found in 90% of patients with CCI. In contrast, Shigemori et al.20) reported that the rostrum and genu were common injury locations21). In the present study, 74% of injuries were located in the anterior two-thirds of the corpus callosum, including the rostrum, genu, and partial parts of the body. Conversely, injuries to the posterior one-third of the corpus callosum were absent, with the exception of 4 patients. Some authors have reported that the reason for differences in the regional frequency of traumatic events is a result of the mechanism of injury and specific anatomical features12781013). Anteriorly, a shorter falx cerebri results in less restrictive movement in the corpus callosum, which may allow transient displacement of brain regions and more subsequent traumatic force. Posteriorly, the falx membrane, which is localized to posterior parts of the corpus callosum, prevents displacement by external forces. These anatomical features, however, do not affect traumatic outcome in all accidents; thus locations of damage to the corpus callosum may differ according to the specific injury121323).
Although the functions of the corpus callosum are not entirely understood, this region is known to act as a bridge between the right and left cerebral hemispheres, and may transfer and coordinate bilateral movement information. Specifically, genu of the corpus callosum was found to transfer neuronal information through the cerebral cortex relating to the control of motor performance, which may be associated with gait disturbance. However, 74% of all patients in this study had injuries located in the anterior two-thirds of corpus callosum, but did not present with any observable difficulties in movement. In this study, 8 patients (50%) had injuries located in the anterior half of corpus callosum. In a recent study, Hofer et al.13) evaluated the microanatomy of the human corpus callosum using diffuse tensor-image-based tractography and distinguished 5 vertical subsegments, containing fibers projecting into the prefrontal, premotor, primary motor, primary sensory, and occipital cortical areas9111323). Their results indicated that transcallosal fibers projecting into the primary motor cortex were localized more posteriorly than previously reported, while primary sensory fibers were located in a posterior location consistent with previous findings. These functional descriptions of the corpus callosum may support the hypothesis that damage to the anterior portion gives rise to moderate clinical symptoms in the absence of radiologic findings18). Theoretically, although the 8 patients with damage in the the posterior half of the corpus callosum should have presented with motor or sensory symptoms, there were no patients who reported these difficulties. Thus, additional studies are needed to explain these anatomical correlations using functional MRI or radiological methods.
Dizziness is a nonspecific presentation that encompasses a wide variety of sensory experiences including lightheadedness, wooziness, faint feeling, feelings of abnormal movement, and rotary sensations of true vertigo in the head. True vertigo most likely indicates vestibular dysfunction of central or peripheral origin. The other dizziness symptoms noted above are difficult to localize in the brain6). Viano et al.22) revealed that orbitofrontal and temporal lobe injury directly resulted in the symptoms of dizziness in mild TBI patients in their 2005 study, in which the finite element analysis applying detailed models of the brain stimulated the brain responses from concussive impacts in National Football League football games. The higher prevalence of dizziness in Group I (37%) compared with that in Group II (12%) in the present study may also suggest the correlation between the high strain in the brain and CCI, though no correlation between dizziness and CCI was found with the current data.
The width of corpus callosum is 7-8 mm, whereas conventional axial brain CT performed with slice thicknesses of 3-5 mm. Only 1 or 2 slices are therefore available to identify lesions in the corpus callosum using brain CT methods. In mild TBI, though CCIs may exist, most instances were small lesions which could not be identified via brain CT. MRI is particularly useful for imaging soft tissue. Thus, using T2-weighted (sagittal plane) brain MRI methods, we could readily recognize lesions, which yielded a high signal intensity within the corpus callosum791011).
The classification of TBI is used to provide a clinical prediction of outcomes in patients with TBI, and dependent on factors such as clinical severity, loss of consciousness, GCS, and posttraumatic amnesia. Because this classification lacks the inclusion of radiologic data, it is difficult to predict clinical outcomes in all patients with TBI710). This is especially true of patients with mild TBI who present with various clinical symptoms but received a diagnosis of cerebral concussion or minor intracranial hemorrhage. Furthermore, the GCS lacks sufficient sensitivity for effective application in many cases of mild TBI-loss of consciousness as an isolated symptom may not be a significant determinant of clinical outcomes. Prolonged neurological symptoms may affect patients to a greater extent than the loss of consciousness for a few seconds. Based on this rationale, we suggest the need for further radiological evaluation in patients with mild TBI when symptoms persist.
There are limitations in this study that should be noted. First, the retrospective design of the study may lead to a selection bias. Secondly, lesions of the corpus callosum did not have a complete correspondence with clinical symptoms. To overcome these limitations, diffusion-tensor imaging is a critical technique that should be used to investigate unexplained neurological symptoms in cases of mild TBI prospectively1825).
In this study, the incidence of CCI in patients with mild TBI who lack posttraumatic parenchymal lesions visible via brain CT scans was approximately 29%. In these cases, patients tended to report more severe headaches and more frequently experienced additional symptoms, such as dizziness and gait disturbance when compared with patients who did not have CCI. We therefore suggest that brain MRI is a useful method to reveal the underlying cause of these posttraumatic symptoms and predict clinical prognosis.
Acknowledgements
This study was supported by BioGreen 21 (PJ01121401) of Rural Development Administration.
References
1. Calvi MR, Beretta L, Dell'Acqua A, Anzalone N, Licini G, Gemma M. Early prognosis after severe traumatic brain injury with minor or absent computed tomography scan lesions. J Trauma. 2011; 70:447–451. PMID: 21307746.

2. Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, et al. Incidence, risk factors and prevention of mild traumatic brain injury : results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004; 43(Suppl):28–60. PMID: 15083870.
3. Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009; 30:3172–3187. PMID: 19241418.

4. Chew BG, Spearman CM, Quigley MR, Wilberger JE. The prognostic significance of traumatic brainstem injury detected on T2-weighted MRI. J Neurosurg. 2012; 117:722–728. PMID: 22860606.

5. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011; 134(Pt 1):73–83. PMID: 21156660.

6. Evans RW. The postconcussion syndrome and the sequelae of mild head injury. Neurol Clin. 1992; 10:815–847. PMID: 1435659.

7. Firsching R, Woischneck D, Klein S, Reissberg S, Döhring W, Peters B. Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir (Wien). 2001; 143:263–271. PMID: 11460914.

8. Gallo A, Rovaris M, Riva R, Ghezzi A, Benedetti B, Martinelli V, et al. Diffusion-tensor magnetic resonance imaging detects normal-appearing white matter damage unrelated to short-term disease activity in patients at the earliest clinical stage of multiple sclerosis. Arch Neurol. 2005; 62:803–808. PMID: 15883269.

9. Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma : review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988; 150:663–672. PMID: 3257624.

10. Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum : MR features. AJNR Am J Neuroradiol. 1988; 9:1129–1138. PMID: 3143234.
11. Godersky JC, Gentry LR, Tranel D, Dyste GN, Danks KR. Magnetic resonance imaging and neurobehavioural outcome in traumatic brain injury. Acta Neurochir Suppl (Wien). 1990; 51:311–314. PMID: 2089925.

12. Gorrie C, Duflou J, Brown J, Gibson T, Waite PM. Extent and distribution of vascular brain injury in pediatric road fatalities. J Neurotrauma. 2001; 18:849–860. PMID: 11565597.

13. Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006; 32:989–994. PMID: 16854598.

14. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury : observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981; 44:285–293. PMID: 6453957.

15. Kampfl A, Franz G, Aichner F, Pfausler B, Haring HP, Felber S, et al. The persistent vegetative state after closed head injury : clinical and magnetic resonance imaging findings in 42 patients. J Neurosurg. 1998; 88:809–816. PMID: 9576247.

16. Lagares A, Ramos A, Pérez-Nuñez A, Ballenilla F, Alday R, Gómez PA, et al. The role of MR imaging in assessing prognosis after severe and moderate head injury. Acta Neurochir (Wien). 2009; 151:341–356. PMID: 19224121.

17. Matsukawa H, Shinoda M, Fujii M, Takahashi O, Yamamoto D, Murakata A, et al. Genu of corpus callosum as a prognostic factor in diffuse axonal injury. J Neurosurg. 2011; 115:1019–1024. PMID: 21780860.

18. Rutgers DR, Fillard P, Paradot G, Tadié M, Lasjaunias P, Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008; 29:1730–1735. PMID: 18617586.

19. Ryberg C, Rostrup E, Stegmann MB, Barkhof F, Scheltens P, van Straaten EC, et al. Clinical significance of corpus callosum atrophy in a mixed elderly population. Neurobiol Aging. 2007; 28:955–963. PMID: 16797787.

20. Shigemori M, Kojyo N, Yuge T, Tokutomi T, Nakashima H, Kuramoto S. Massive traumatic haematoma of the corpus callosum. Acta Neurochir (Wien). 1986; 81:36–39. PMID: 3728089.

21. Tokutomi T, Hirohata M, Miyagi T, Abe T, Shigemori M. Posttraumatic edema in the corpus callosum shown by MRI. Acta Neurochir Suppl. 1997; 70:80–83. PMID: 9416285.

22. Viano DC, Casson IR, Pellman EJ, Zhang L, King AI, Yang KH. Concussion in professional football : brain responses by finite element analysis : part 9. Neurosurgery. 2005; 57:891–916. discussion 891-916PMID: 16284560.

23. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989; 112(Pt 3):799–835. PMID: 2731030.

24. Yanagawa Y, Sakamoto T. Significance of minor traumatic lesions in focal head injuries. J Clin Neurosci. 2011; 18:520–523. PMID: 21315605.

25. Yasuno F, Matsuoka K, Kitamura S, Kiuchi K, Kosaka J, Okada K, et al. Decision-making deficit of a patient with axonal damage after traumatic brain injury. Brain Cogn. 2014; 84:63–68. PMID: 24316983.

26. Yuan F, Ding J, Chen H, Guo Y, Wang G, Gao WW, et al. Predicting outcomes after traumatic brain injury : the development and validation of prognostic models based on admission characteristics. J Trauma Acute Care Surg. 2012; 73:137–145. PMID: 22743383.
Fig. 1
Witelson's classification23). The corpus callosum is one of the few white matter tracts that can be discretely identified by conventional MRI. In 1989, Witelson delineated distinct callosal areas in a midsagittal cross-section based on specific mathematical proportions with respect to the anterior (A) and posterior (P) axis. He divided the midsagittal corpus callosum geometrically into segments defined as the following : the anterior third (region I, containing fibers projecting into prefrontal, premotor and supplementary motor regions), anterior midbody (region II, with callosal motor fiber bundles), posterior midbody (region III, with somaesthetic, posterior parietal projections), isthmus (region IV, with posterior parietal, superior temporal projections), and splenium (region V, with the occipital, inferior temporal projections).
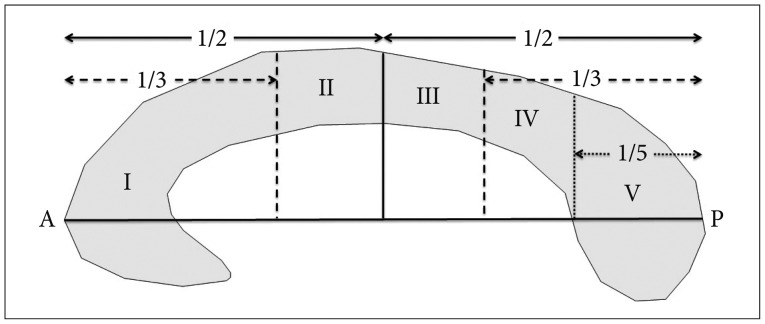
Fig. 2
MRI and CT images of TBI patient with CCI. A : Upon admission, no abnormal findings are visible on brain CT imaging. B : T2-weighted sagittal MRI revealed a previously unidentified high signal change in region III of the corpus callosum (arrowhead, images captured 3 days following CT imaging). C : Follow-up imaging 3 months after initial MRI depicting a low-density lesion on the body of the corpus callosum (arrowhead, potentially indicating tissue loss following CCI). CCI : corpus callosum injury.
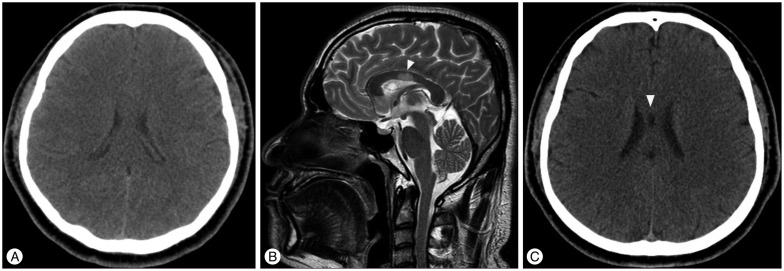
Table 2
Summary of 16 patients with CCI as assessed by brain MRI
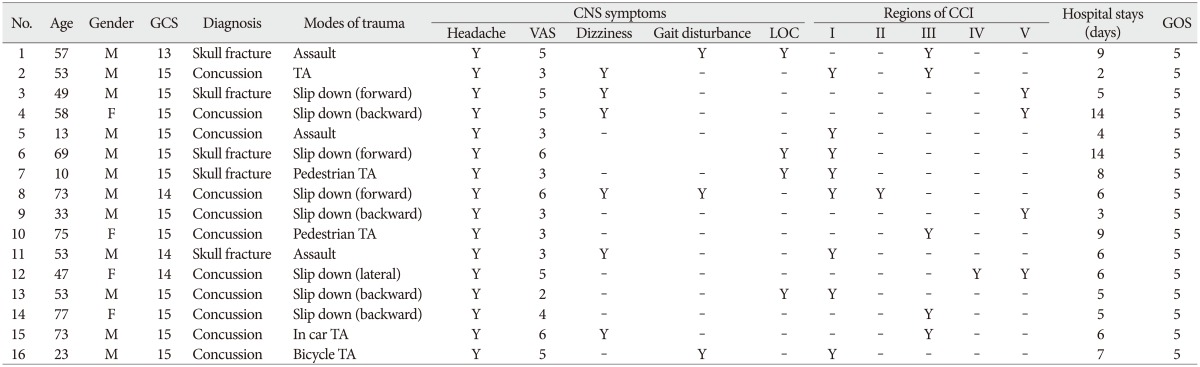
CCI : corpus callosum injury, No : patient number, TA : traffic accident, GCS : Glasgow coma scale, VAS : visual analogue scale, LOC : loss of consciousness, GOS : Glasgow outcome scale, Region I : the anterior third, Region II : the anterior midbody, Region III : the posterior midbody, Region IV : the isthmus, Region V : the splenium, Y : applicable, - : not applicable




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download