Abstract
Objective
The aim of this study was to explore the immunity in rats transplanted with adipose-derived mesenchymal stem cells (ADSCs) and acellular nerve (ACN) for repairing sciatic nerve defects.
Methods
ADSCs were isolated from the adipose tissues of Wistar rats. Sprague-Dawley rats were used to establish a sciatic nerve defect model and then divided into four groups, according to the following methods : Group A, allogenic nerve graft; Group B, allograft with ACN; Group C, allograft ADSCs+ACN, and Group D, nerve autograft.
Results
At the day before transplantation and 3, 7, 14, and 28 days after transplantation, orbital venous blood of the Sprague-Dawley rats in each group was collected to detect the proportion of CD3+, CD4+, and CD8+ subsets using flow cytometry and to determine the serum concentration of interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) using enzyme-linked immunosorbent assay (ELISA). At each postoperative time point, the proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, TNF-α, and IFN-γ in group C were all near to those in group B and group D, in which no statistically significant difference was observed. As compared with group A, the proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, TNF-α, and IFN-γ were significantly reduced in group C (p<0.05).
Peripheral nerve defect is a common disabling disease clinically. To date, the most common and effective treatment for it is autologous nerve transplantation. However, there are still a lot of disadvantages existing in this treatment, such as the donor site dysfunction, neuroma formation, limited sources, the size being difficult to match and so on3). Allogenic transplantation has a wide range of sources but great immunological rejection. The histocompatibility complex existing on the surface of the nerve cells contributes to the antigens of the immunological rejection1415). Hudson et al.6) reported that chemical extraction methods can remove the membrane and medulla sheath of nerve tissues, so as to eliminate the antigens including the histocompatibility complex, which can greatly reduce the immune response of the nerve graft and retain the base pipe membrane and lamellar structure of the nerve cells, providing a good bionic channel for nerve regeneration. However, a body of experiments shows that a simple transplantation with acellular nerve (ACN) cannot achieve the similarly satisfactory clinical results with autologous nerve graft. It probably results from the removal of Schwann cells22). Adipose-derived mesenchymal stem cells (ADSCs) are a class of stem cells with multipotent differentiation capacity with little or no immunogical rejection, because their surfaces express less or no major histocompatibility complex (MHC)-II. They can be oriented to differentiate into Schwann cells and then promote the peripheral nerve regeneration, which has been proved by in vivo and in vitro studies79141625). In this study, an artificial neural repair of rat sciatic nerve defects constructed with ADSCs and ACN was used to observe the proportion of T lymphocyte subsets and cytokine changes, so as to explore the immunological rejection of transplantation with ADSCs and ACN and provide an immunological evidence for its clinical application.
32 healthy male Sprague-Dawley (SD) rats and 12 healthy male Wistar rats, weighing 150 g to 200 g, were offered by the Experimental Animal Center of Wenzhou Medical University, Zhejiang, China. SD rats were used as the recipients of nerve transplantation and Wistar rats were used as the donors of ADSCs and ACN.
After Wistar rats (n=8) were intraperitoneally anesthetized with 10% chloral hydrate (0.3 mL/100 g), the bilateral inguinal fat pads of the rats were isolated and cut into pieces. Then double volumes of 0.1% collagenase I were added into the tissue fragments and incubated at 37℃ for 60 min (shaking it in every 10 minutes). The digestion was stopped by the equal volume of DMEM/F12 medium containing 15% fetal bovine serum (FBS). After filtered with a 200 mesh filter, the filtrate was centrifuged for 10 minutes at 1000 r/min. Removing the supernatant, the precipitate was resuspended in DMEM/F12 containing 15% FBS. 1×105 cells were seeded in the 25 cm2 flasks and incubated in a CO2 incubator. The medium was changed in every 48h. As the cells adhered and grew to 80-90% of confluence, they were passaged in a ratio of 1 : 3. The third passage was observed under an inverted microscope and then harvested for detection of the surface markers CD44, CD90, and CD45 by flow cytometry.
ACN was also donated by the 8 Wistar rats after they donated the adipose tissues. Under the sterile conditions, about 2.0 cm bilateral sciatic nerves were cut out. Following the methods described by Hudson et al.6), the nerves were successively treated with distilled water, sulfobetaine-10 (SB-10), sulfobetaine-16 (SB-16) and Triton-200 to extract the acellular nerve. Part of the nerves was used for hematoxylin-eosin staining (HE) staining and electron microscopic scanning.
ADSCs were prepared into 1.0×107/mL with DMEM/F12 medium containing 15% FBS and 1% penicillin-streptomycin. Then 10 µL ADSCs suspension were injected into the ACN with micro-syringe, every 2 µL and every other 3 mm to each injection point, then incubated in the 5% carbon dioxide incubator at 37℃ overnight to construct artificial nerve.
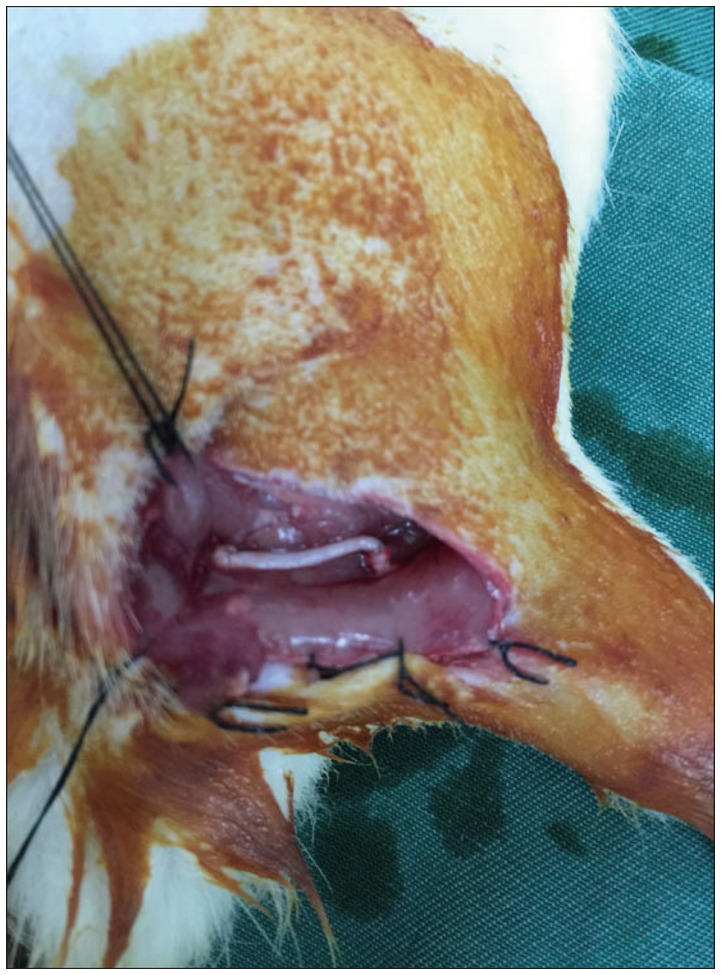
32 SD rats were intraperitoneally anesthetized with 10% chloral hydrate (0.4 mL/100 g) and then 1.5 cm of the right sciatic nerve was cut out under the piriformis to make sciatic nerve defect model. Then the rats were randomly divided into four groups (n=8) and underwent immediate nerve transplantation, secured with a 8-0 suture according to the following programs : group A was treated with allogenic nerve; group B was treated with ACN extracted chemically; group C was treated with the artificial nerves constructed with ADSCs and ACN (Fig. 1), and group D was treated with nerve autograft.
At the day before the transplantation and 3, 7, 14, and 28 days after the transplantation, orbital venous blood of SD rats in each group was collected to detect the proportion of CD3+, CD4+, and CD8+ subsets using flow cytometry. 7 µL anti-rat CD3, CD4, and CD8 antibodies were added into 50 µL orbital venous blood, respectively. After the cells were mixed thoroughly and incubated in dark for 30 min, 600 µL solution A (0.12% formic acid, prepared before use) was added into each tube and mix thoroughly. 15 s later, solution B (NaCl 14.5 g, Na2SO4 31.3 g, and Na2CO3 6.0 g in 1000 mL distilled water, prepared before use) was also added. After mixed, cells were centrifuged at 1500 rpm for 5 min. The cells were washed with PBS buffer once again and centrifuged for 5 minutes. Then the labeled cells were resuspended in 500 µL PBS buffer and detected on a FACSArial digital cell sorter (BD Biosciences Pharmingen, San Jose, CA, USA).
The serum concentration of IL-2, TNF-α, and IFN-γ of rats in each group at the day before the transplantation and 3, 7, 14, and 28 days after the transplantation were determined using the Ready-SET-Go ELISA kits (Lianke Bio Co. Ltd., Hangzhou, China), following the manufacturer's instructions.
ADSCs showed fibroblast-like cells under an inverted microscope. Their phenotypes were CD45 (-), 90 (+), and 44 (+) (Fig. 2).
The ACN extracted chemically showed milky white and intact shape. As stained with hematoxylin-eosin, ACN revealed intact nerve membrane but its components had disappeared. As scanned by a scanning electron microscopy, medulla sheath and Schwann cells could not be observed any more. However, the three-dimensional structure of the acellular nerve cells was kept better, as observed with a transmission electron microscopy (Fig. 3).
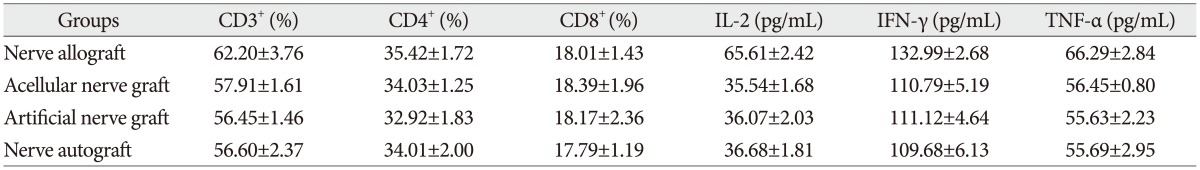
The proportion of CD3+, CD4+, and CD8+ subsets of group C at 3, 7, 14, and 28 days after the transplantation increased slightly, as compared with those before the transplantation, but there was no significant difference (p>0.05). They were also not significantly different from those of group B and group D (p>0.05). The proportion of CD3+, CD4+, and CD8+ subsets of group A began to increase at 3 days after the transplantation, but showed no significant difference from those before the transplantation. However, they increased continually and reached a peak at 7-14 days after the transplantation and then decreased at 28 days after the transplantation, showing a significant difference from those before the transplantation. The postoperative proportion of CD3+, CD4+, and CD8+ subsets of group A were also significantly different from those of group B, C, and D (p<0.05, Table 1, 2, 3, 4, 5) (Fig. 4).
The serum concentration of IL-2, TNF-α, and IFN-γ of group C at 3, 7, 14, and 28 days after the transplantation showed similar increasing trend with the proportion of T lymphocyte subsets as compared with those before the transplantation (p>0.05) or those of group B and group D (p>0.05, respectively). IL-2, IFN-γ, and TNF-α in group A began to increase at 3 days after the transplantation, in which IL-2 level showed significant difference (p<0.05) but IFN-γ and TNF-α showed no significant difference (p>0.05), as compared with those before the transplantation. IL-2, IFN-γ, and TNF-α also increased to a peak at 7 to 14 days after the transplantation and then declined after 28 days, which were all significantly different from those before transplantation (p<0.05). Similarly, they were also significantly higher than those of group B, C, and D (p<0.05).
The counts and types of lymphocyte subsets in vivo are sensitive indicators to reflect the graft rejection and immune status in vivo. Normally, the number of T lymphocyte and its subsets are relatively stable in the peripheral blood. However, if there were immune dysfunction or graft rejection happening, the number of T lymphocyte would increase accordingly. CD4+ T cells are helper/inducer T lymphocytes and CD8+ T cells are inhibitor/killer T cells, whose balance is an important indicator to reflect the transplantation immunity. CD3 antigen is expressed on the surface of all of the mature T cells, which is also an important indicator for the body's transplantation immunity20). Artificial nerves constructed with Schwann cells that orientedly induced from acellular nerve and ADSCs by tissue engineering are non-vascularized before they are transplanted. Its transplantation differs from the allograft rejection of vascularized solid organ transplantation, which is a typical cell-mediated rejection. The effector cells of cell-mediated immunological response include cytotoxic T cells (CTL) and T helper cells (THL). CTL cells can bind to the surface MHC-I antigen directly to kill the antigen. T helper cells can be divided into Th1 and Th2. Th1 cells mediate the cellular immunity by producing IL-2, IFN-γ, and TNF-α. Thus, these cytokines can be good indicators for cellular immunity8242628). Th2 cells are mainly involved in the proliferation of B cells. Therefore, in this study, the proportion of CD3+, CD4+, and CD8+ cells and the serum concentration of IL-2, IFN-γ, and TNF-α were regarded as the indicators for exploring the transplantation immunity of co-graft of Schwann cells induced from ADSCs and acellular nerve to repair the sciatic nerve defect of rats. At 2-3 days after transplantation, there may be an increased inflammation, and then it would be relieved at 5-10 days after transplantation, followed by a two-week immunosuppressive phases. At 3-4 weeks after transplantation, the recipient was in the recovery period. Thus, the indicators were tested at the day before the transplantation (baseline), 7, 14, and 28 days after the transplantation in this study.
At the various time points after transplantation with artificial nerves constructed with ADSCs and chemically extracted acellular nerve, the proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, IFN-γ, and TNF-α were not significantly different from those of the rats with nerve autograft, indicating there was no apparent allograft rejection no matter at the early phage or the recovering stage of the immune response in acute transplantataion. At 7-14 days after nerve allograft, the proportion of CD3+, CD4+, CD8+ subsets and the serum concentration of IL-2, IFN-γ, and TNF-α were significantly increased to a peak, which was consistent with the previous report of Tung et al.23) that nerve allograft can induce obvious Th1 effect. Chemical extraction can remove the antigen and other substances of Schwann cells to become acellular nerves. It can significantly reduce the immunogenicity of the implantation121727). In addition to the less amount of the implantation, systemic immunological response will not be induced. ADSCs do not express the surface MHC-II molecules and T lymphocyte costimulatory molecules. Thus, they can escape the killing effect of cytotoxic T lymphocytes and NK cells1019) and be able to survive in vivo to induce a host immune tolerance, which may have immunomodulatory effects on T lymphocytes219), B cells221) and natural killer cells1121) but rarely cause immunological rejection513). Since neither acellular nerve nor ADSCs have significant immunogenicity, co-graft of acellular nerve and ADSCs does not cause immunological rejection. Kuo et al.11) found that ADSCs can inhibit the proliferation of T cells. The infusion of donor ADSCs in combination with other immunosuppressive therapy can significantly prolong the survival time of allogenic limb transplantation and reduce the tissue rejection. However, if the topical application of allogeneic ADSCs would affect the immunological response of the host is rarely reported yet. In this study, the acellular nerve graft and the co-graft with acellular nerve and ADSCs were not significantly different in immunological rejection, suggesting that topical application of ADSCs has no obvious effect on the host immunological response. The weakness of this experiment is that we didn't trace the transplanted ADSCs to show it survived in the host or differentiated into Schwann-like cells? However, there were reports show the transplanted ADSCs survived for a long time. Santiago et al.18). reported that human uASCs enhanced peripheral nerve regeneration and decreased muscular atrophy when transplanted into poly-caprolactone nerve guides to repair a 6-mm nerve gap in athymic rats; cells survived up to 12 weeks after transplantation but did not differentiate into Schwann cells.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Zhejiang Province, China (Y2100253) and the Science and Technology Program of Wenzhou City, Zhejiang Province, China (Y20130203).
References
1. Bain JR, Mackinnon SE, Hudson AR, Falk RE, Falk JA, Hunter DA. The peripheral nerve allograft : an assessment of regeneration across nerve allografts in rats immunosuppressed with cyclosporin A. Plast Reconstr Surg. 1988; 82:1052–1066. PMID: 3264409.
2. Comoli P, Ginevri F, Maccario R, Avanzini MA, Marconi M, Groff A, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008; 23:1196–1202. PMID: 18029377.

3. de Ruiter GC, Spinner RJ, Yaszemski MJ, Windebank AJ, Malessy MJ. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 2009; 20:91–105. viiPMID: 19064182.

4. Gao X, Wang Y, Chen J, Peng J. The role of peripheral nerve ECM components in the tissue engineering nerve construction. Rev Neurosci. 2013; 24:443–453. PMID: 23907421.

5. Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010; 15:86–91. PMID: 19949335.

6. Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004; 10:1641–1651. PMID: 15684673.

7. Jiang L, Zhu JK, Liu XL, Xiang P, Hu J, Yu WH. Differentiation of rat adipose tissue-derived stem cells into Schwann-like cells in vitro. Neuroreport. 2008; 19:1015–1019. PMID: 18580571.

8. Kang HG, Zhang D, Degauque N, Mariat C, Alexopoulos S, Zheng XX. Effects of cyclosporine on transplant tolerance : the role of IL-2. Am J Transplant. 2007; 7:1907–1916. PMID: 17617853.

9. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007; 207:267–274. PMID: 17761164.

10. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T cell responses to allogeneic human mesenchymal stem cells : immunogenicity, tolerance, and suppression. J Biomed Sci. 2005; 12:47–57. PMID: 15864738.

11. Kuo YR, Chen CC, Goto S, Lee IT, Huang CW, Tsai CC, et al. Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model. Plast Reconstr Surg. 2011; 128:661e–672e.

12. Kvist M, Sondell M, Kanje M, Dahlin LB. Regeneration in, and properties of, extracted peripheral nerve allografts and xenografts. J Plast Surg Hand Surg. 2011; 45:122–128. PMID: 21682608.

13. Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012; 21:2770–2778. PMID: 22621212.

14. Liu GB, Cheng YX, Feng YK, Pang CJ, Li Q, Wang Y, et al. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci. 2011; 7:592–596. PMID: 22291793.

15. Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001; 107:1419–1429. PMID: 11335811.

16. Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, et al. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012; 18:1264–1272. PMID: 22332955.

17. Rovak JM, Bishop DK, Boxer LK, Wood SC, Mungara AK, Cederna PS. Peripheral nerve transplantation : the role of chemical acellularization in eliminating allograft antigenicity. J Reconstr Microsurg. 2005; 21:207–213. PMID: 15880301.

18. Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009; 18:145–158. PMID: 19499703.

19. Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010; 71:267–274. PMID: 20384870.

20. Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998; 795:44–54. PMID: 9622591.

21. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006; 24:74–85. PMID: 16099998.

22. Sun XH, Che YQ, Tong XJ, Zhang LX, Feng Y, Xu AH, et al. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol. 2009; 29:347–353. PMID: 18987968.

23. Tung TH, Mohanakumar T, Mackinnon SE. TH1/TH2 cytokine profile of the immune response in limb component transplantation. Plast Reconstr Surg. 2005; 116:557–566. PMID: 16079692.

24. Velásquez SY, García LF, Opelz G, Alvarez CM, Süsal C. Release of soluble CD30 after allogeneic stimulation is mediated by memory T cells and regulated by IFN-γ and IL-2. Transplantation. 2013; 96:154–161. PMID: 23857000.

25. Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L, Chen J, et al. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett. 2012; 514:96–101. PMID: 22405891.

26. Yu X, Jiang Y, Lu L, Gong X, Sun X, Xuan Z, et al. A crucial role of IL-17 and IFN-γ during acute rejection of peripheral nerve xenotransplantation in mice. PLoS One. 2012; 7:e34419. PMID: 22479627.

27. Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010; 31:5312–5324. PMID: 20381139.

28. Zimmerer JM, Horne PH, Fiessinger LA, Fisher MG, Pham TA, Saklayen SL, et al. Cytotoxic effector function of CD4-independent, CD8(+) T cells is mediated by TNF-α/TNFR. Transplantation. 2012; 94:1103–1110. PMID: 23222736.

Fig. 2
The expression of CD44 (A), CD45 (B), and CD90 (C) of ADSCs determined using flow cytometry. ADSCs : adipose-derived mesenchymal stem cells.
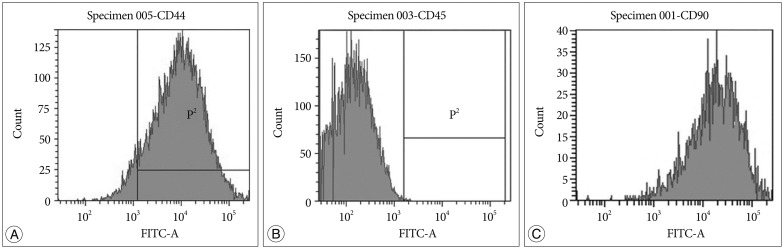
Fig. 3
Longitudinal section of acellular nerve displayed an intact three-dimensional structure (scanning electron microscopy, 5000×). IL-2 : interleukin-2, TNF-α : tumor necrosis factor-α, IFN-γ : interferon-γ.
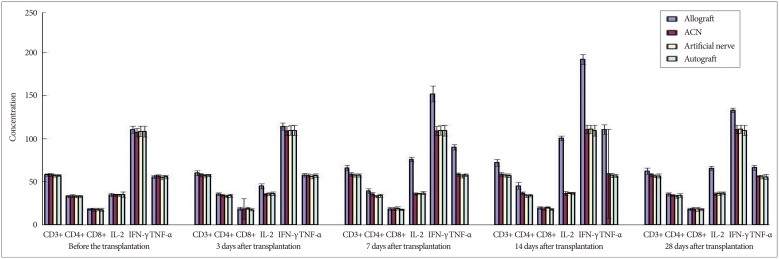
Fig. 4
The proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, IFN-γ, and TNF-α. IL-2 : interleukin-2, TNF-α : tumor necrosis factor-α, IFN-γ : interferon-γ.
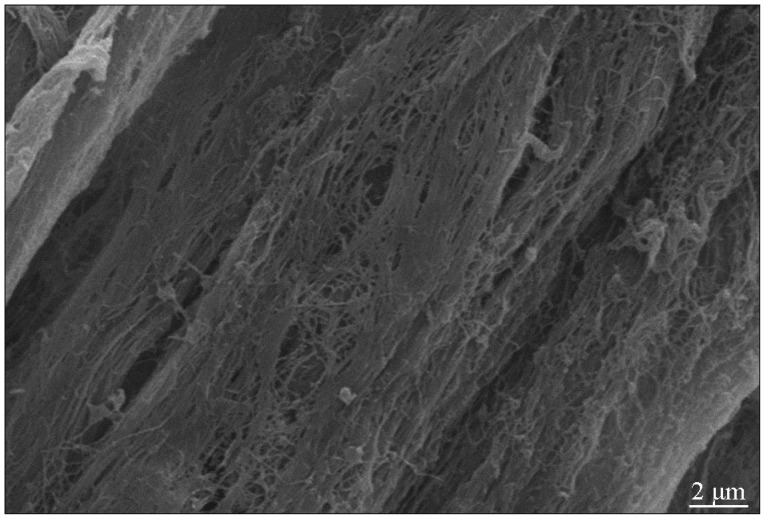
Table 1
The proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, IFN-γ, and TNF-α at the day before transplantation
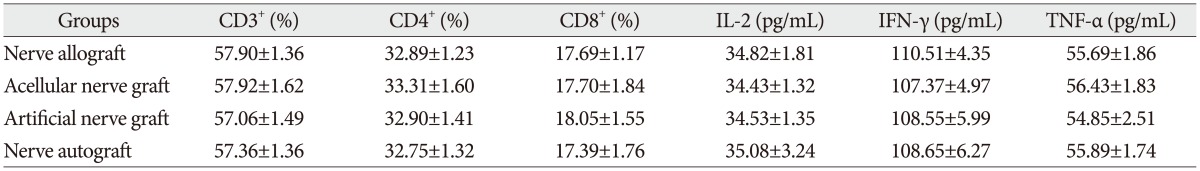
Table 2
The proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, IFN-γ, and TNF-α at 3 days after transplantation
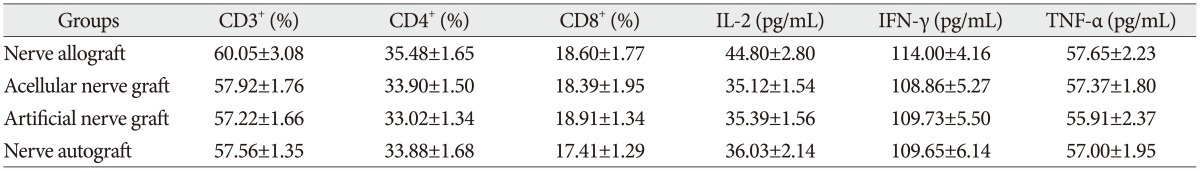
Table 3
The proportion of CD3+, CD4+, and CD8+ subsets and the serum concentration of IL-2, IFN-γ, and TNF-α at 7 days after transplantation
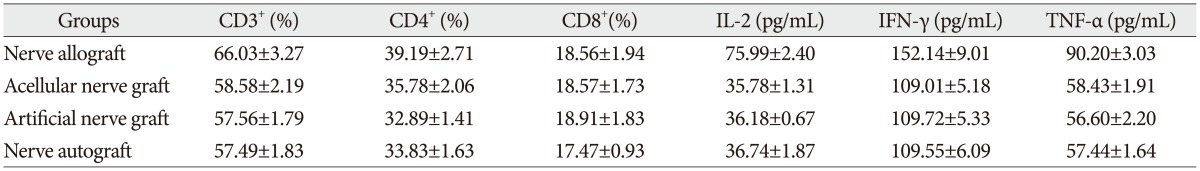




 PDF
PDF ePub
ePub Citation
Citation Print
Print


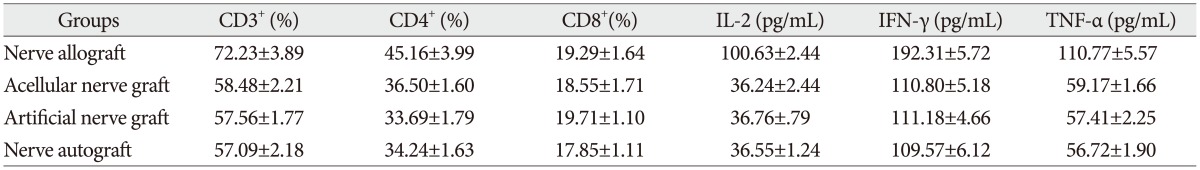
 XML Download
XML Download