Abstract
We report two rare cases of spinal intraosseous schwannoma (SIS) with sustained myelopathy symptoms and provide an updated review regarding SIS in the literature. A 71-year-old man experienced right lumbocrural pain and gait disturbance accompanied with paresthesia and right leg weakness. Imaging examinations revealed a mass with lesions in L4 vertebral body causing bone destruction and spinal cord compression. Complete resection of the well-demarcated tumor and posterior fusion were performed. A 54-year-old female reported bilateral gait disturbance, paresthesia, and numbness without weakness, and imaging revealed a posterior mass from T9 causing spinal cord compression and bone erosion. The tumor was completely separated from the spinal nerve root. The tumors from both patients were confirmed as schwannomas. Tumor recurrence was not observed at the 2-4 year follow-up. Although rare, SIS should be considered during differential diagnosis and can affect treatment planning. SIS symptoms vary depending on tumor location, and fusion is frequently necessary for spinal reconstruction after complete tumor resection.
Schwannoma (neurilemmoma) is a benign tumor that arises mainly in sensory nerve sheaths24). Intraosseous lesions are rare, accounting for less than 0.2% of primary bone tumors3), and the majority are located in the mandible and sacrum68916). Other reported sites include the ulna, humerus, femur, tibia, ribs, patella, scapula, maxilla, the small bones of the hands, and vertebral bodies2). Spinal intraosseous schwannoma (SIS) was first reported in 19645). SIS is an extremely rare lesion, and the diagnosis remains unclear, mostly because of its origin. This is the focus of controversy between some authors, whereas the pathological diagnosis and surgical treatment are quite similar. Correctly diagnosing SIS without resection is difficult given the broad range of symptoms. However, appropriate diagnosis is important to properly plan surgical intervention. Magnetic resonance imaging (MRI) with contrast can be useful in preoperatively diagnosing SIS, and histopathology is very informative for resected specimens. Here, we report two cases of intraosseous schwannoma involving the body of L4 and the posterior structures of T9 that were diagnosed following myelopathic symptoms.
A 71-year-old man experienced right lumbocrural pain and gait disturbance accompanied by paresthesia and right leg weakness for 6 months. Neurologic examination revealed impaired right leg motor function (grade 3/5) with diminished feeling on the right side caudally from the lumbar L4 sensory dermatome; however, his nerve reflexes were normal. Enhanced magnetic resonance imaging (MRI) (Fig. 1) showed a mass with lesions in the vertebral body (L4) and spinal canal compressing the lumbar spinal cord. Computed tomography (CT) scan revealed a slowly growing tumor with severe vertebrae destruction (Fig. 2A, arrow). Piecemeal resection and decompression were performed. After total laminectomy and facetectomy of L3-5, a well-demarcated tumor was exposed extending into the spinal canal (Fig. 2B, arrow) without nerve involvement or dural adhesions. The spine was stabilized with pedicle screws and rods after the tumor was completely resected. Histological characteristic of the tumor revealed Antoni A and B tissue (Fig. 2C) and overexpression of S-100 protein (Fig. 2D), which confirmed a diagnosis of intraosseous schwannoma without originating nerve remnants. Intraoperative fluoroscopy revealed successful internal fixation (Fig. 3A). Postoperative images taken at the 2-year follow-up showed no obvious evidence of recurrence and general bony fusion (Fig. 3B, C, D), and the patient's gait and sensation in the right lower extremity showed good recovery.
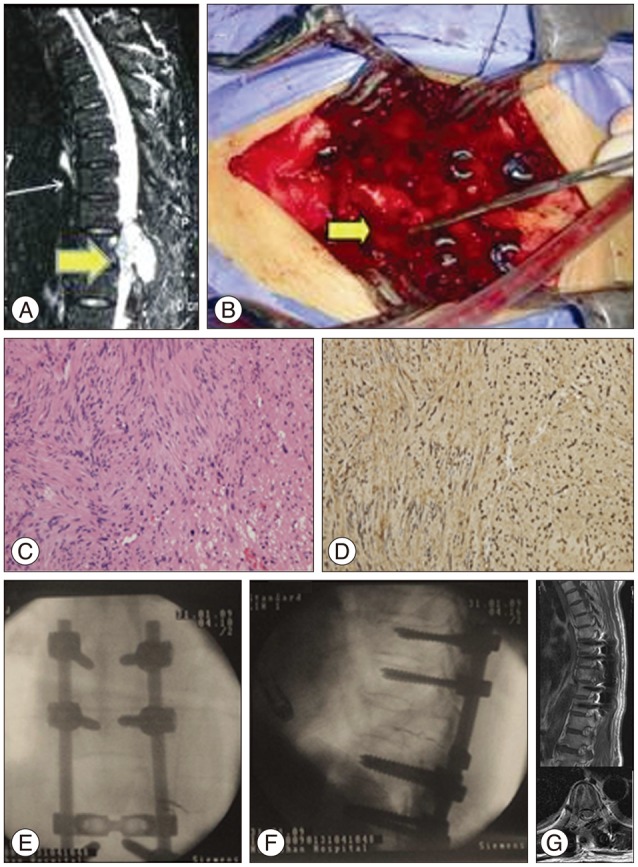
A 54-year-old female reported a 4-month history of gait disturbance and paresthesia of both lower extremities. Physical examination revealed numbness in both legs without obvious weakness (grade 5/5); nerve reflexes were normal. MRI (Fig. 4A) showed a mass (arrow) that appeared to originate from the posterior elements of T9 and extended into the spinal canal and paravertebral areas, extruding the spinal cord. T9 vertebrae bone erosion was observed on CT. The imaging results led to a differential diagnosis of primary benign/malignant bone tumor or metastatic tumor. The tumor was completely separated and surgically resected from the spinal nerve root with a clear border, and a posterior fusion with allograft bone was performed to stabilize the spine. Pathological characteristics of the tumor confirmed a benign schwannoma (Fig. 4C, D). Intraoperative fluoroscopy demonstrated successful internal fixation in the proper position for posterior interbody fusion (Fig. 4E, F). According to the MRI images at the 4-year follow-up, there was no obvious sign of reoccurence with relieved gait and sensation disturbance (Fig. 4G).
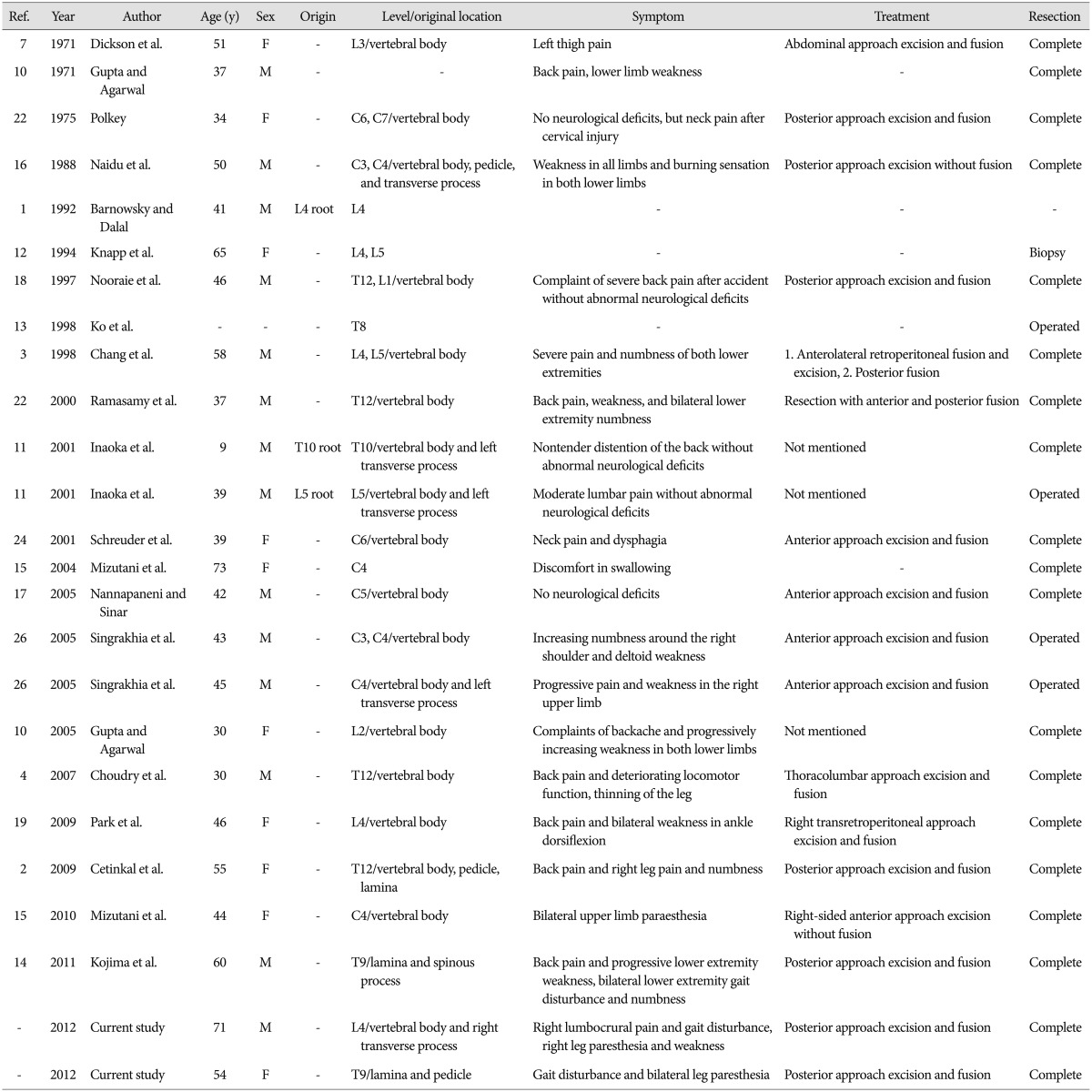
Although SIS is quite rare, a number of cases have been published in the last 30 years123471011121314151617181921222427). We looked at images in the cited articles and found classic pre- and intra-operative images of SIS to ensure that there was no connection between the tumor and nerve tissue, but the photos were not always convincing. Our report aims to provide a clear definition of SIS and a review of this rare disease. Because the symptoms associated with SIS can vary depending on their location in the spinal cord and because they can overlap with those manifested in other conditions, incorrect diagnosis remains a problem. A relatively complete summary of SIS cases described from 1971-2012 is shown in Tables 1, 2. Though the cases reported by Barnowsky and Dalal1) and Inaoka et al.11) showed that Schwannoma originated from nerve root. It was defined as SIS by Park et al.19). The majority of reports do not hold this view, and we determined those cases were probably intraosseous invasions of extraosseous nerve sheath tumors. It is known that neurilemmomas can involve bone by three possible mechanisms : 1) an extraosseous tumor causing secondary bone erosion; 2) a tumor arising centrally within the bone; and 3) tumor origination in the nutrient canal followed by growth into a dumbbell-shape that enlarges the spinal canal36911222324). Of these, only the second mechanism could occur with intraosseous neurilemmoma; the small nerves that give rise to these tumors have been described in the human vertebrae2025). In other words, the intraosseous origin of schwannoma must be nerves within bones that are free from adjacent neural tissue.23) This view is supported by most reports in the SIS literature.211)
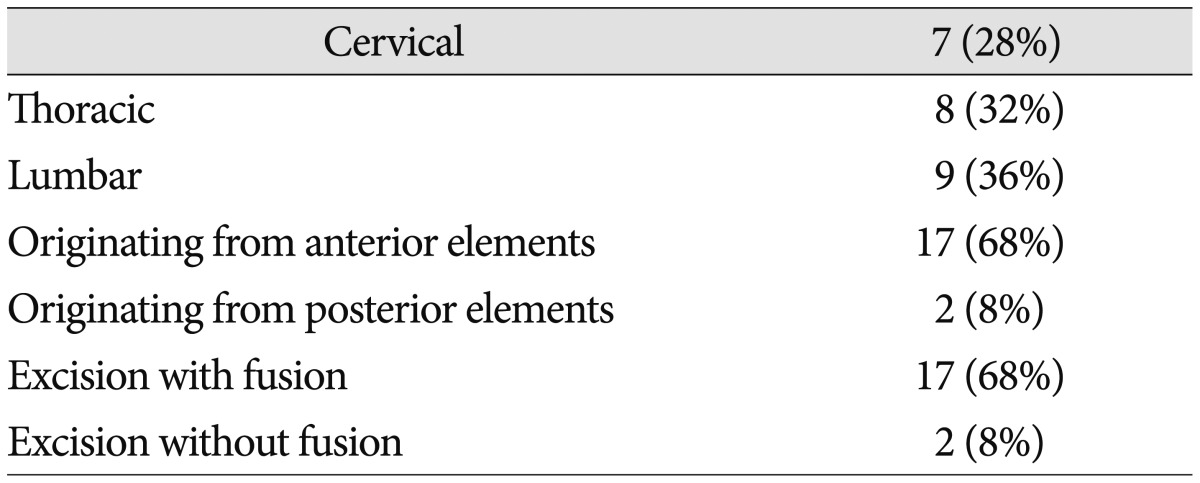
Symptoms vary among SIS patients. Because most of these tumors enlarge slowly,19) the patient's history may be considerably long, and most experience pain (14/25, 56%) depending on the tumor location.24) Neurological compression symptoms develop when the tumor perforates the bone cortex and causes spinal cord protrusion, but specific symptoms can differ depending on the level of the lesion.22) SISs are most commonly found in the lumbar region (38%), followed by thoracic (32%) and cervical (28%), which is different from the results reported by Park et al.19)
Radiological findings in SIS can also vary considerably, and differential diagnosis includes ruling out solitary myeloma, chordoma, chondrosarcoma, giant cell tumor, angioma, and aneurysmal bone cyst. SISs are sometimes found to primarily occupy the intraosseous region with or without extravertebral and spinal canal involvement, and a hollowed out vertebral body with a single, thin, bulging cortex perforation is suggestive of intraosseous origin.3) Generally, intraosseous schwannoma appears on radiological images as a lytic defect with bone erosion lacking new periosteal bone formation and calcification/ossification, although a narrow sclerotic zone may be present between the tumor and bone324). Vertebral intraosseous schwannomas gradually increase in size, resulting in pedicle and vertebral body erosion that widens the foramen and vertebral scalloping.
Histological conformation is mandatory for a diagnosis of SIS. Proliferation of slender spindle cells with oval nuclei and focal palisading nuclei (Antoni A) and degenerated hypocellular areas (Antoni B) with hemosiderin deposition and thrombosed blood vessels are suggestive of schwannoma3). SISs are not histologically different from schwannomas that develop elsewhere, even at the ultrastructural level16), but the histological features of intraosseous neurilemmomas may be obscured in highly cellular lesions with subtle Antoni types A and B patterns11). Both types have long durations16) and similar degenerative characteristics, including perivascular hyalinization, calcification, and cystic degeneration.
The gold standard for benign bone tumors is marginal resection. Unfortunately, this is often difficult to achieve in SISs, which have both intra- and extraosseous components that invade adjacent structures, including nerve roots, spinal cord, and paravertebral tissue16). This is usually addressed with adequate curettage beyond the original margins of the tumor without local adjuvant, which can result in long-term relief without a high recurrence rate24). Despite their tendency to be benign, SISs often result in spinal instability due to severe vertebral body invasion, and fusion with bone graft is required in approximately 68% of cases.
We report two cases of SIS, which is a rare differential diagnosis for intraosseous tumor. Proper diagnosis requires radiological tests, gross intraoperative findings, and postoperative histological results. Symptoms vary depending on tumor location, and fusion is necessary to stabilize the spine after the tumor is completely excised.
References
1. Barnowsky L, Dalal R. Extradural schwannoma manifested as an expansile vertebral lesion. AJR Am J Roentgenol. 1992; 159:1352–1353. PMID: 1442424.

2. Cetinkal A, Atabey C, Kaya S, Colak A, Topuz AK. Intraosseous schwannoma of thoracic 12 vertebra without spinal canal involvement. Eur Spine J. 18(Suppl 2):2009; 236–239. PMID: 19255790.

3. Chang CJ, Huang JS, Wang YC, Huang SH. Intraosseous schwannoma of the fourth lumbar vertebra : case report. Neurosurgery. 1998; 43:1219–1222. PMID: 9802868.
4. Choudry Q, Younis F, Smith RB. Intraosseous schwannoma of D12 thoracic vertebra : diagnosis and surgical management with 5-year follow-up. Eur Spine J. 16(Suppl 3):2007; 283–286. PMID: 17082954.

5. Cohen DM, Dahlin DC, Maccarty CS. Apparently solitary tumors of the verteral column. Mayo Clin Proc. 1964; 39:509–528. PMID: 14163176.
6. de la Monte SM, Dorfman HD, Chandra R, Malawer M. Intraosseous schwannoma : histologic features, ultrastructure, and review of the literature. Hum Pathol. 1984; 15:551–558. PMID: 6724574.

7. Dickson JH, Waltz TA, Fechner RE. Intraosseous neurilemoma of the third lumbar vertebra. J Bone Joint Surg Am. 1971; 53:349–355. PMID: 5546707.

8. Ellis GL, Abrams AM, Melrose RJ. Intraosseous benign neural sheath neoplasms of the jaws. Report of seven new cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1977; 44:731–743. PMID: 270070.
9. Gordon EJ. Solitary intraosseous neurilemmoma of the tibia : review of intraosseous neurilemmoma and neurofibroma. Clin Orthop Relat Res. 1976; (117):271–282. PMID: 1277675.
10. Gupta SP, Agarwal A. Intraosseous neurilemmoma of L2 vertebra--a case report. Indian J Pathol Microbiol. 2005; 48:367–369. PMID: 16761755.
11. Inaoka T, Takahashi K, Hanaoka H, Aburano R, Tokusashi Y, Matsuno T, et al. Paravertebral neurinoma associated with aggressive intravertebral extension. Skeletal Radiol. 2001; 30:286–289. PMID: 11407721.

12. Knapp TR, Struk DW, Munk PL, Bainbridge TC, Bhimji SD, Poon PY. Tumour of a peripheral nerve sheath with invasion of the lumbar spine. Can Assoc Radiol J. 1994; 45:469–472. PMID: 7982111.
13. Ko SF, Lee TY, Lin JW, Ng SH, Chen WJ, Hsieh MJ, et al. Thoracic neurilemomas : an analysis of computed tomography findings in 36 patients. J Thorac Imaging. 1998; 13:21–26. PMID: 9440835.
14. Kojima M, Seichi A, Yamamuro K, Inoue H, Kimura A, Hoshino Y. Intraosseous schwannoma originating from the posterior column of the thoracic spine. Eur Spine J. 20(Suppl 2):2011; S153–S156. PMID: 20496036.

15. Mizutani A, Yokota N, Kawaji H, Yamaguchi-Okada M, Miyagawa T, Namba H. Intraosseous schwannoma of the cervical vertebral body : a case report and review of the literature. Br J Neurosurg. 2010; 24:604–646. PMID: 20632880.
16. Naidu MR, Dinakar I, Rao KS, Ratnakar KS. Intraosseous schwannoma of the cervical spine associated with skeletal fluorosis. Clin Neurol Neurosurg. 1988; 90:257–260. PMID: 3197354.

17. Nannapaneni R, Sinar EJ. Intraosseous schwannoma of the cervical spine. Br J Neurosurg. 2005; 19:244–247. PMID: 16455526.

18. Nooraie H, Taghipour M, Arasteh MM, Daneshbod K, Erfanie MA. Intraosseous schwannoma of T12 with burst fracture of L1. Arch Orthop Trauma Surg. 1997; 116:440–442. PMID: 9266062.

19. Park SC, Chung SK, Choe G, Kim HJ. Spinal intraosseous schwannoma : a case report and review. J Korean Neurosurg Soc. 2009; 46:403–408. PMID: 19893734.

20. Pedersen HE, Blunck CF, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerve (sinu-vertebral nerves); with an experimental study of their functions. J Bone Joint Surg Am. 1956; 38-A:377–391. PMID: 13319400.
21. Polkey CE. Intraosseous neurilemmoma of the cervical spine causing paraparesis and treated by resection and grafting. J Neurol Neurosurg Psychiatry. 1975; 38:776–781. PMID: 1102628.

22. Ramasamy P, Shackleford I, Al Jafari M. Schwannoma of t12 vertebra : case report and review of literature. Sarcoma. 2000; 4:185–190. PMID: 18521301.

23. Samter TG, Vellios F, Shafer WG. Neurilemmona of bone. Report of 3 cases with a review of the literature. Radiology. 1960; 75:215–222. PMID: 14441281.
24. Schreuder HW, Veth RP, Pruszczynski M, Lemmens JA, van Laarhoven EW. Intraosseous schwannoma (neurilemmoma) of the cervical spine. Sarcoma. 2001; 5:101–103. PMID: 18521311.

25. Sherman MS. The nerve of bone. J Bone Joint Surg Am. 1963; 45:522–528.
26. Singrakhia MD, Parmar H, Maheshwari M, Fehlings M. Cervical schwannoma presenting as an expansile vertebral body lesion : report of two cases with a technical note on the surgical management. Surg Neurol. 2006; 66:192–196. discussion 196PMID: 16876626.

Fig. 1
Preoperative MR images from case 1 (71-year-old male), showing an abnormality in the L4 vertebra body. A : T1-weighted imaging. The tumor is isointense compared with the spinal cord. B : Gd-enhanced T1-weighted imaging showing irregular enhancement.
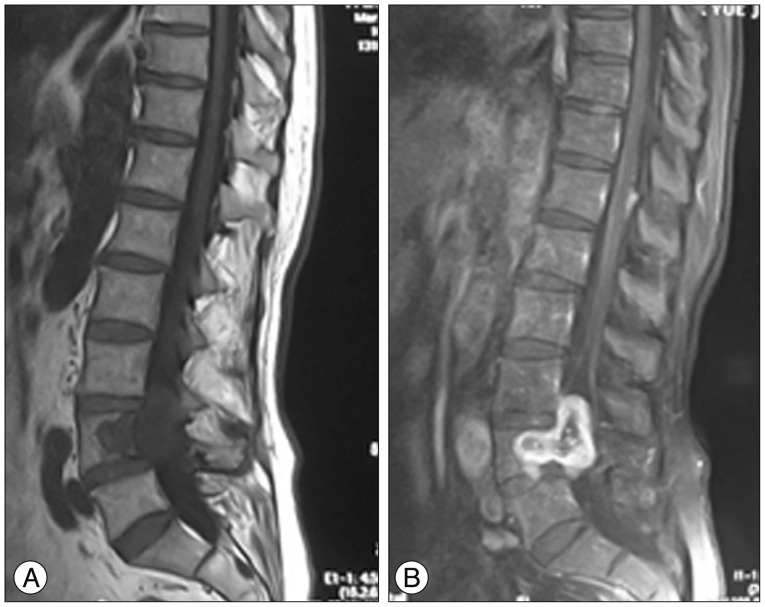
Fig. 2
Case 1, a 71-year-old male with L4 intraosseous schwannoma. A : High-signal intensity (arrow) on preoperative CT showing a tumor compressing the spinal cord. B : Intraoperative image showing the tumor (arrow) extending to the spinal canal. C : Histology revealed hypercellular (Antoni A) and hypocellular (Antoni B) areas, indicating a typical schwannoma (H&E staining, ×200). D : Immunohistochemistry showing S-100 protein over-expression (brown color, ×200).
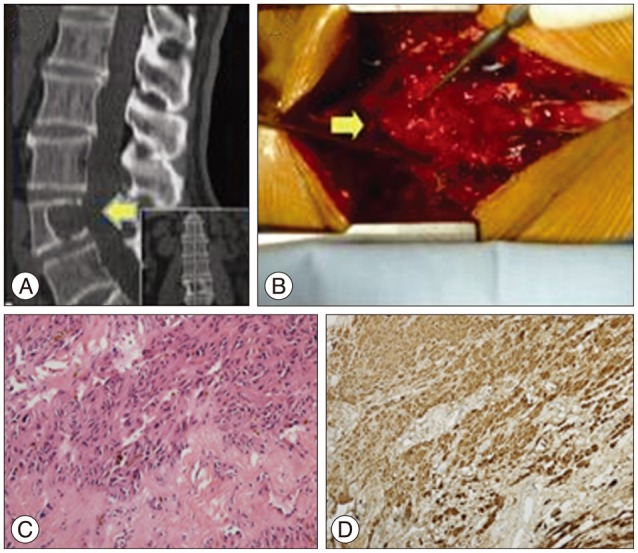
Fig. 3
Radiology images of case 1 from immediately after 2 years follow-up. A : Intraoperative fluoroscopy showed proper internal fixation (top : anteroposterior, bottom : lateral). B : Postoperative X-ray images showing that general fusion was realized without any internal fixation dislocation (top : anteroposterior, bottom : lateral). C : There was no reoccurrence observed on the MRI at the 2-year follow-up (top : lateral, bottom : coronal). D : Follow-up CT images taken 2 years later revealed that general fusion was achieved without any internal fixation dislocation (top : lateral, bottom : coronal).
Fig. 4
Case 2, a 54-year-old female with T9 intraosseous schwannoma. A : T2-weighted MR imaging showing mixed-intensity tumor tissue (arrow). B : The tumor originated from posterior T9 without nerve root involvement (arrow), and we performed total piecemeal resection followed by posterior spinal reconstruction. C : Histology revealed hypercellular (Antoni A) and hypocellular (Antoni B) areas, indicating a typical schwannoma (H&E stain; ×200). D : Immunohistochemistry revealed S-100 protein overexpression (brown color, ×200). E and F : Intraoperative fluoroscopy demonstrated successful internal fixation (E : anteroposterior, F : lateral). G : There was no obvious sign of reoccurence at 4 year postoperatively according to the MRI images (top : lateral position, bottom : coronal).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download