Abstract
Moyamoya disease is a unique cerebrovascular disorder characterized by idiopathic progressive stenosis at the terminal portion of the internal carotid artery (ICA) and fine vascular network. The aim of this review is to present the clinical application of quantitative digital subtraction angiography (QDSA) in pediatric moyamoya disease. Using conventional angiographic data and postprocessing software, QDSA provides time-contrast intensity curves and then displays the peak time (Tmax) and area under the curve (AUC). These parameters of QDSA can be used as surrogate markers for the hemodynamic evaluation of disease severity and quantification of postoperative neovascularization in moyamoya disease.
Moyamoya disease is a unique cerebrovascular disorder characterized by idiopathic progressive stenosis at the terminal portion of the internal carotid artery (ICA) and fine vascular network, the so-called "moyamoya vessels"6). Surgical treatment for ischemic-type moyamoya disease is reliable with regard to establishing an adequate collateral circulation in the ischemic brain to prevent cerebral infarction. Indirect revascularization is the most widely used treatment to induce angiogenesis in pediatric moyamoya disease6).
MRI and MR angiography are widely used for the diagnosis and follow-up of moyamoya disease. However, these conventional techniques do not fully demonstrate the complex hemodynamics in moyamoya disease9). The conventional digital subtraction angiography (DSA) is still viable for diagnosing moyamoya disease, and Suzuki grading remains the reference standard for evaluating disease severity5811). It is known that postoperative neovascularization or angiogenesis induces disappearance of moyamoya vessels and progression of the steno-occlusive changes in the ICA. As a result, surgical treatment apparently seems to accelerate the increase in the angiographic stage2511). However, accurate longitudinal changes in neovascularization have not been fully elucidated. Recently the use of color-coded parametric quantitative DSA (QDSA) has been reported in various vascular conditions114). This review presents the clinical application of QDSA for the quantitative evaluation of neovascularization in pediatric moyamoya disease.
Conventional angiographic examinations were performed using a biplane angiography suite (AXIOM-Artis; Siemens, Forchheim, Germany). A 4 Fr angiocatheter was placed at the level of the C4 vertebral body for DSA of the common carotid artery (CCA). The imaging parameter was six frames per second for the DSA throughout the acquisition series until maximal opacification of the superior sagittal sinus was achieved. Six milliliter of a 60% diluted contrast medium (340 mg/mL) was injected into the patients at a rate of 4 mL/s using a power injector for CCA. Next, we performed ICA and external carotid artery (ECA) angiography using 3-5 mL of contrast medium with 1.5-2.0 mL/s flow rate.
Severity of steno-occlusive changes in the ICA was classified into six angiographic stages as defined by Suzuki and Takaku8). : Stage I, narrowing of the carotid bifurcation only; Stage II, dilation of the main cerebral arteries with the appearance of moyamoya vessels; Stage III, partial disappearance of the anterior and middle cerebral arteries with increased moyamoya vessels around the circle of Willis; Stage IV, advanced steno-occlusive changes in the ICA, with the anterior cerebral artery (ACA) and middle cerebral artery (MCA) traced very dimly or in a completely different shape; Stage V, absence of the ACA and MCA with further reduction of moyamoya vessels; and Stage VI, blood supply only from the ECA and an almost complete disappearance of moyamoya vessels. The extent of neovascularization through ECA was evaluated as follows : grade 1, one-third or less of the MCA distribution only; grade 2, between one-third and two-thirds of the MCA distribution; grade 3, greater than two-thirds of the entire MCA distribution4).
The DSA images were color-coded using the post processing software Syngo iFlow (Siemens Healthcare, Forchheim, Germany), based on the X-ray attenuation along the angiogram7). The software provides a single colored-composite image that shows the path of the movement of contrast medium though the vessels in time. For quantitative analysis, regions of interest (ROI) were placed on the colored image. The software calculated relative opacity data per ROI per time point. It provides time-contrast intensity curves for the ROIs and then displays the peak time (Tmax) and area under the curve (AUC) (Fig. 1). Total relative perfusion was estimated by AUC calculated as the sum of the relative densities at each time point divided by the frame rate. QDSA has been used to access the peritherapeutic hemodynamics in the various vascular disorders including steno-occlusive arterial disease in an angiographic suite313).
QDSA increases the conspicuity of subtle blood flow changes and enables the quantitative monitoring of hemodynamics in the angiographic suite. Although angiographic grading (Suzuki grading) is still the reference standard for the severity of moyamoya disease, it does not represent the hemodynamic status of the whole brain parenchyma1012). QDSA could provide a more sensitive parameter of disease severity than conventional DSA. In addition, QDSA could provide the cerebral circulation time and AUC as a surrogate hemodynamic marker in patients with moyamoya disease.
In the preoperative angiography of moyamoya disease, the delay time of maximal opacification (Tmax) between the ICA and the MCA can be measured, and Tmax can be correlated with the hemodynamic parameters obtained from dynamic susceptibility-weighted perfusion-weighted magnetic resonance imaging (DSC-PWI). For example, increased Tmax can be observed in the symptomatic hemisphere that shows delayed time to peak (TTP) on DSC-PWI (Fig. 1C).
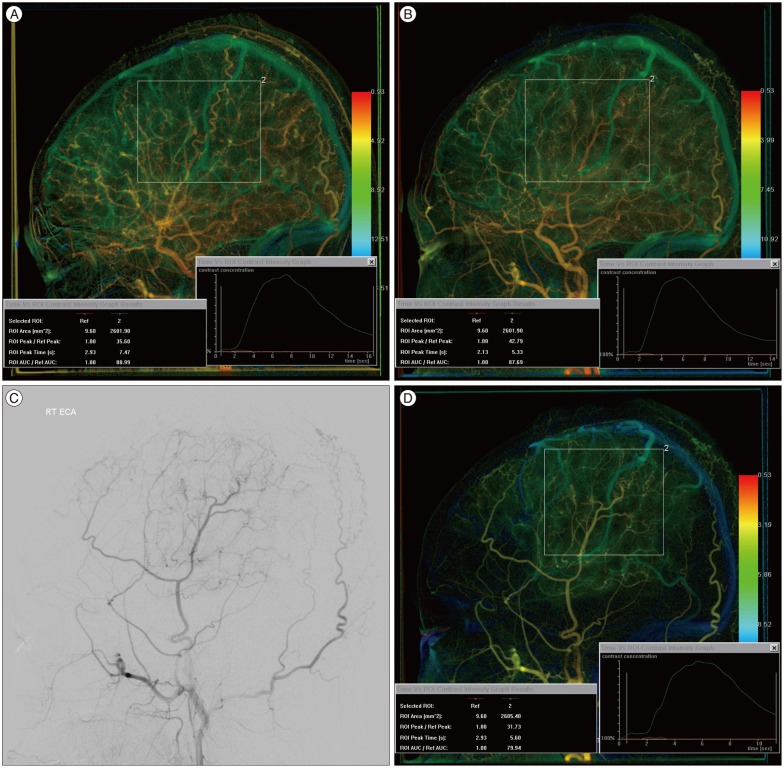
For evaluation of neovascularization in postoperative moyamoya disease, two ROIs were placed : one on the proximal CCA and the other on the frontoparietotemporal area corresponding to the bony flap in the lateral view. Compared to the preoperative angiography, QDSA demonstrates postoperative neovascularization both quantitatively and qualitatively. For example, ROI analysis demonstrates a higher AUC value and a short Tmax compared to preoperative QDSA in the area of neovascularization (Fig. 2). On postoperative ECA angiography, the Tmax and AUC can be measured directly in the area of neovascularization (Fig. 2D).
For the longitudinal comparison of QDSA data, the injection rate and total amount of the contrast medium should be controlled during angiographic examination because it might result in different shapes and waveform of time-density curves and Tmax, respectively. Standardizing the DSA procedures throughout the study could have minimized these variations. QDSA has no additional invasive procedure or radiation. However, cerebral angiography in children is an invasive procedure and procedure-related complications may occur as a result.
In conclusion, QDSA provides qualitative and quantitative information in pediatric patients with moyamoya disease using conventional angiographic data. It is a potentially good surrogate marker for the hemodynamic evaluation of disease severity and quantification of postoperative neovascularization in moyamoya disease.
References
1. Hung SC, Liang ML, Lin CF, Lin CJ, Guo WY, Chang FC, et al. New grading of moyamoya disease using color-coded parametric quantitative digital subtraction angiography. J Chin Med Assoc. 2014; 77:437–442. PMID: 25028291.

2. Kim SJ, Son TO, Kim KH, Jeon P, Hyun SH, Lee KH, et al. Neovascularization precedes occlusion in moyamoya disease : angiographic findings in 172 pediatric patients. Eur Neurol. 2014; 72:299–305. PMID: 25323466.

3. Lin CJ, Hung SC, Guo WY, Chang FC, Luo CB, Beilner J, et al. Monitoring peri-therapeutic cerebral circulation time : a feasibility study using color-coded quantitative DSA in patients with steno-occlusive arterial disease. AJNR Am J Neuroradiol. 2012; 33:1685–1690. PMID: 22499839.

4. Matsushima T, Inoue T, Ikezaki K, Matsukado K, Natori Y, Inamura T, et al. Multiple combined indirect procedure for the surgical treatment of children with moyamoya disease. A comparison with single indirect anastomosis and direct anastomosis. Neurosurg Focus. 1998; 5:e4. PMID: 17112207.
5. Robertson RL, Burrows PE, Barnes PD, Robson CD, Poussaint TY, Scott RM. Angiographic changes after pial synangiosis in childhood moyamoya disease. AJNR Am J Neuroradiol. 1997; 18:837–845. PMID: 9159360.
6. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 360:1226–1237. PMID: 19297575.

7. Strother CM, Bender F, Deuerling-Zheng Y, Royalty K, Pulfer KA, Baumgart J, et al. Parametric color coding of digital subtraction angiography. AJNR Am J Neuroradiol. 2010; 31:919–924. PMID: 20167651.

8. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969; 20:288–299. PMID: 5775283.
10. Togao O, Mihara F, Yoshiura T, Tanaka A, Noguchi T, Kuwabara Y, et al. Cerebral hemodynamics in Moyamoya disease : correlation between perfusion-weighted MR imaging and cerebral angiography. AJNR Am J Neuroradiol. 2006; 27:391–397. PMID: 16484417.
11. Yamada I, Matsushima Y, Suzuki S. Childhood moyamoya disease before and after encephalo-duro-arterio-synangiosis : an angiographic study. Neuroradiology. 1992; 34:318–322. PMID: 1528443.

12. Yun TJ, Cheon JE, Na DG, Kim WS, Kim IO, Chang KH, et al. Childhood moyamoya disease : quantitative evaluation of perfusion MR imaging--correlation with clinical outcome after revascularization surgery. Radiology. 2009; 251:216–223. PMID: 19332853.

13. Zhang Q, Xu R, Sun Q, Zhang H, Mao J, Shan T, et al. : Exploring the value of using color-coded quantitative DSA evaluation on bilateral common carotid arteries in predicting the reliability of intra-ascending aorta flat detector CT-CBV maps. AJNR Am J Neuroradiol. 2015; [Epub ahead of print].
14. Zhang XB, Zhuang ZG, Ye H, Beilner J, Kowarschik M, Chen JJ, et al. Objective assessment of transcatheter arterial chemoembolization angiographic endpoints : preliminary study of quantitative digital subtraction angiography. J Vasc Interv Radiol. 2013; 24:667–671. PMID: 23489772.

Fig. 1
A 4-year-old boy with left hemiparesis. A : Right internal carotid artery (ICA) angiography shows steno-occlusive change of the distal ICA with basal collateral formation (Suzuki type III). B : Color-coded quantitative DSA (QDSA) demonstrates slow flow (green to blue color) in the right middle cerebral artery (MCA) territory compared to the PCA territory (yellow to orange color). ROI analysis of the MCA territory depicts a time-contrast intensity curve using which Tmax and AUC can be calculated. C : Perfusion MRI of the same patient depicts delayed time to peak in the right MCA territory.
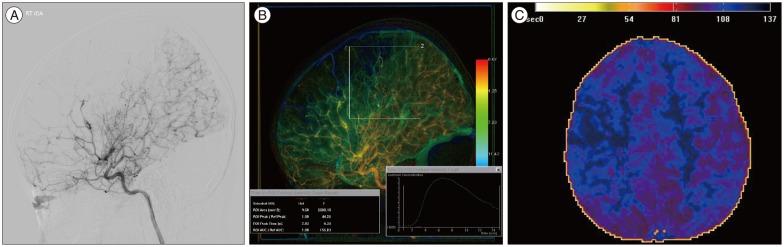
Fig. 2
A 9-year-old girl with left hemiparesis. A and B : Compared to the preoperative quantitative DSA (QDSA) of the right common carotid artery (CCA) (A), postoperative QDSA of the right CCA (B) demonstrates an improved delay of Tmax (7.47 s→5.33 s) and an increased mean AUC. C : Right external carotid artery (ECA) angiography after encephalo-duro-arterio-synangiosis (EDAS) shows good neovascularization (grade 2). D : QDSA of the right ECA angiography after EDAS provides Tmax and AUC of the neovascularization.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download