Abstract
Chronic subdural hematomas mainly occur amongst elderly people and usually develop after minor head injuries. In younger patients, subdural collections may be related to hypertension, coagulopathies, vascular abnormalities, and substance abuse. Different techniques can be used for the surgical treatment of symptomatic chronic subdural hematomas : single or double burr-hole evacuation, with or without subdural drainage, twist-drill craniostomies and classical craniotomies. Failure of the brain to re-expand, pneumocephalus, incomplete evacuation, and recurrence of the fluid collection are common complications following these procedures. Acute subdural hematomas may also occur. Rarely reported hemorrhagic complications include subarachnoid, intracerebral, intraventricular, and remote cerebellar hemorrhages. The causes of such uncommon complications are difficult to explain and remain poorly understood. Overdrainage and intracranial hypotension, rapid brain decompression and shift of the intracranial contents, cerebrospinal fluid loss, vascular dysregulation and impairment of venous outflow are the main mechanisms discussed in the literature. In this article we report three cases of different post-operative intracranial bleeding and review the related literature.
Chronic subdural hematomas (CSH) are frequently encountered in neurosurgical practice. Risk factors include head trauma, advanced-age, frequent falls, coagulopathies, and therapeutic anticoagulation1012). Cerebrospinal fluid (CSF) shunts and overshunting conditions, and the age-related increase of subdural space and subarachnoid cisterns may also promote CSH formation1124). Furthermore, the cause of CSH is unknown in 29-38% of cases1224). Spontaneous subdural hematomas occurring in younger patients1) are supposed to be due to severe arterial hypertension, intracranial hypotension7), CSF leaks, repeated Valsalva maneuvers13), vascular abnormalities-like arteriovenous malformations (AVM) and aneurysms20)-dural sinus thrombosis21), neoplasms, infections, coagulopathies and alcohol or cocaine abuse18).
Several techniques are used for the surgical treatment of symptomatic subdural hematomas12) : single burr-hole craniectomy under local anesthesia, with or without positioning a subdural drainage system, double burr-hole evacuation technique, twist-drill craniostomy and formal craniotomy. Neuroendoscopic techniques have also been proposed for multiloculated or septated haematomas14), but are rarely recommended. Although craniotomy has been widely used in combination with inner membranectomy27), a higher mortality rate was demonstrated. For this reason, less invasive procedures such as subdural evacuating port systems, are currently spreading over28).
Mori and Maeda24) reported in 2001 that post-operative acute subdural hematoma (2.6%) and tension pneumocephalus (0.8%) were the most common complications in CSH surgery. In this series, acute subdural hematoma is attributed to fresh bleeding from the scalp wound. Residual subdural fluid collections and failure of the brain to re-expand may also occur after these surgical procedures. Other post-operative complications include CSH recurrence, seizures and subdural empyema2730).
Cortical hyperemia beneath the hematoma, subarachnoid hemorrhage (SAH), supratentorial intracerebral, intraventricular, and remote cerebellar hemorrhages (RCHs) are rarely observed. In a large series of 1000 CSH12), 4 cases of post-operative intracranial bleeding were described (0.4%). Other authors report an incidence range between 0.2 and 4%824). In our case series, 385 CSH were operated in the Neurosurgery Unit of the University Hospital of Varese, from January 2006 to December 2013. Unusual post-operative hemorrhagic events occurred in 3 patients, with an incidence of 0.78%. The causes of these uncommon complications remain unexplained. Overdrainage, rapid brain decompression and shift of the intracranial contents, massive CSF loss, venous outflow impairment and vascular dysregulation with blood flow increase, are the mechanisms currently debated.
In case 1 we report the clinical findings regarding a 62-years-old man. He presented with a fortnight history of headache and progressive weakness of the left limbs. He did not suffer from any previous pathology and he did not refer substance abuse. No history of trauma was detected. The neurologic examination demonstrated a slight left hemiparesis. Computed tomography (CT) revealed a right chronic subdural hematoma with mass effect on brain parenchyma and mild shift of the midline structures (Fig. 1). Consequently, we proceeded with the surgical evacuation of the right subdural hematoma via a single parietal burr-hole craniectomy. At the end of the operation, a subdural drainage tube was positioned, open to gravity. A few hours after the procedure the patient developed intense headache and mental confusion, without neurological focal deficits. Arterial blood pressure was 150/90 mm Hg. A large amount of recent reddish blood was found in the drainage system, which contained 650 mL of fluid. We decided to immediately close the drainage and perform a CT scan. This revealed a diffuse SAH localized in the basal cisterns, bilaterally in the sylvian fissures, and over the cerebral surface (Fig. 2). No residual fluid collection, acute bleeding or severe pneumocephalus were found in the cavity of the hematoma. CT-angiography and cerebral angiography excluded the presence of cerebral aneurysms and AVM. The drainage system was kept closed for the next two days. The patient was left in supine position and the fluid intake was increased to 1.5 L per day. Headache was successfully treated with analgesics. After two days, a CT scan showed normal brain expansion and the re-absorption of subarachnoid blood without further complications. Therefore the drainage tube was removed. The patient's conditions gradually improved, the headache decreased and after five days he was discharged without neurological deficits. The one-month follow-up showed that the patient was in good conditions. Accordingly, he returned to his work and leisure activities. Head CT was normal.
In case 2 we report the findings regarding an 85-years-old man with a history of hypertension, ischemic heart disease, cardiac arrhythmia and chronic obstructive pulmonary disease (COPD). He was admitted to our department due to mental confusion, disorientation and dizziness which had occurred ten days back. Previous mild head trauma due to an accidental fall was reported. Neurological evaluation confirmed mental confusion, spatial disorientation and gait imbalance, without focal deficits. The CT scan revealed a sizeable right chronic-subacute subdural hematoma with mass effect and midline shift (Fig. 3). Blood thinners (clopidogrel 75 mg per day) were discontinued 7 days before surgery. The patient underwent a single parietal burr-hole craniectomy and the right CSH was uneventfully evacuated. A subdural drainage apparatus was positioned, open to gravity. The day after the patient suddenly developed arterial hypertension (200/110 mm Hg), intense headache and vomit. We found a large amount of fluid (500 mL) in the drainage bag. The device was closed and a CT scan was performed. This revealed the presence of SAH in the right sylvian fissure, and a small contralateral occipital intraparenchymal hemorrhage (Fig. 4). The patient was kept in recumbent position for two days and the fluid intake was increased to 1.5 L per day. Normal arterial pressure values were restored with medical therapy and the headache gradually decreased. Two days after, a CT scan did not show other complications. The drainage system was then removed and after five days the patient was discharged without neurological deficits. The one-month follow-up showed the patient was in good conditions. The CT scan of the head revealed complete resolution of the previous hemorrhagic findings.
In case 3 we report the findings regarding an 80-years-old man with a history of alcohol abuse, arterial hypertension and ischemic heart disease. He was admitted to our hospital due to headache and dizziness. He reported frequent falls in the previous month and a minor head trauma. The CT scan showed a right CSH with mass effect and a thin liquid collection on the left side (Fig. 5). The neurologic evaluation revealed mental confusion and weakness of the left limbs. Antiplatelet therapy (aspirin 100 mg per day) was discontinued 5 days before surgery. The evacuation of the subdural hematoma was performed with a single parietal burr-hole craniectomy on the right side. A subdural drainage catheter was positioned, open to gravity, without evidence of complications. The following day we observed a worsening of the neurologic conditions with mental confusion and lethargy. A large amount of fluid (600 mL) was encountered in the drainage system. Arterial pressure was 170/100 mm Hg. The post-operative CT scan (Fig. 6) showed frontal bilateral pneumocephalus and a small left temporal intracerebral hemorrhage. No acute bleedings or increase in the left hemispheric hygroma were found. Additionally, thin blood layers along the tentorium and within the cerebellar folia were demonstrated. The drainage tube was removed, the patient was left in recumbent position and the fluid intake was increased to 1.5 L per day. The CT findings gradually resolved and did not require surgical exploration. The neurologic evaluation revealed a slight postural instability and for this reason the patient was addressed to physical therapy. At two-month follow-up, the patient was able to walk unaided and the control CT scan was normal.
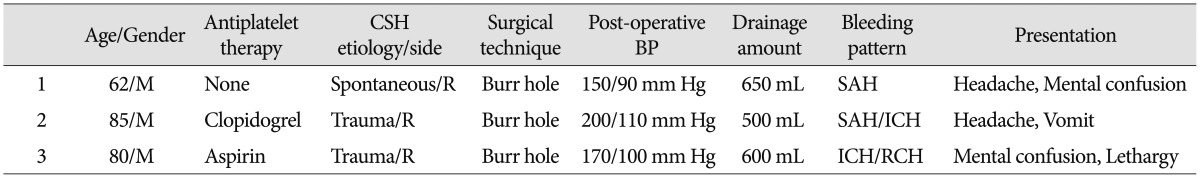
Three different bleeding patterns occurred after CSH evacuation are described in our report. The characteristics of patients are summarized in Table 1. The first case is a diffuse bilateral SAH localized in the basal and sylvian cisterns and over the cerebral convexity. The patient was a healthy 62 years old man, without history of trauma, coagulation disorders or substance abuse. He developed a slight post-operative hypertension with headache. No aneurysms or AVM were detected by the cerebral angiography. Further causes of SAH include angiographically occult vascular malformations, coagulation disorders, pituitary apoplexy, sickle cell anemia, drug consumption, cerebral artery dissection and vasculitides, but none of these was demonstrated. Spontaneous, "sine materia" subarachnoid hemorrages517) have also been described and even this unlikely and quite benign condition has to be considered.
Miyazaki et al.22) reported in 2004 the only case of post-operative SAH of unknown origin after CSH drainage. The patient was a 52-years-old man with a history of cerebral infarctions being treated with anticoagulants, complaining of headache without evidence of head trauma. The authors mainly related this hemorrhagic complication to peri-operative hypertension and anticoagulant therapy. Other discussed mechanisms are the rapid shift of the cerebral hemisphere after hematoma evacuation, overdrainage of the subdural space and the hyperperfusion syndrome. This condition was described in the elderly by Ogasawara et al.25). According to this study vascular autoregulation is impaired due to long-term brain compression by CSH, and a rapid decrease of intracranial pressure occurs after the drainage of the hematoma. The consequent hemodynamic changes can lead to hyperperfusion and cortical hyperemia, resulting in the rupture of a weak subarachnoid vessel. Moreover, peri-operative mean arterial pressure was significantly higher in patients exhibiting this syndrome.
Standard bore (19 Fr), open to gravity drainage systems are routinely utilized in our unit, without suction bulb. Catheters are inserted in the subdural space and usually removed 48-72 hours after surgery. We usually perform a CT scan on first post-operative day. Patients are kept in supine position until the drainage is removed. In case 1, a large amount of fluid was found in the patient's drainage bag soon after surgery, and fresh blood traces were remarkable. We suppose that the combination of rapid parenchymal shift and diffuse cortical hyperemia were responsible for developing of SAH in our patient. The former may be related to the prompt expansion of a "healthy" brain after sudden decompression, whereas the latter has probably been promoted by hypertension and hyperperfusion.
The second case is an 85-years-old man with a history of hypertension, ischemic heart disease, cardiac arrhythmia, and COPD. He developed an important hypertensive condition with headache and vomit the day after burr-hole trephination. Head CT revealed a right sylvian SAH and a contralateral occipital hematoma.
Several cases of intracerebral hemorrhages following CSH evacuation have been reported, but the pathogenesis of this dangerous complication remains unclear. Modesti et al.23) reported a 5% incidence of post-operative intracerebral hematomas and many of these patients did not survive or were left with severe disability. Hemorrhage into previously undetected contusions, sudden increase in cerebral blood flow and arterial hypertension, combined with faulty local autoregulation, were accounted as pathogenic mechanisms. The authors concluded that these factors led to vascular damage and the consequent bleeding.
Kim et al.19) reported in 2013 the case of a massive and fatal intracerebral hemorrhage after CSH drainage. In their case the patient was a young man previously treated with a low dose of aspirin. The patient developed post-operative disseminated intravascular coagulation. Parenchymal bleeding was ascribed to overdrainage of the subdural space with rapid parenchymal shift and to the coagulation disorder. The authors state that the loss of equilibrium among the tightly regulated coagulation factors can lead either to hypercoagulable states, with microthrombosis and ischemia, or to hypocoagulable states with possible onset or progression of hemorrhagic lesions. In addition, Spetzler et al.29) obtained experimental evidence of the loss of CO2 reactivity and autoregulation in the ischemic hemisphere of animals. The loss of vascular autoregulation might be also related to the gap between pre-operative cerebral blood flow (reduced by long term compression due to impeded venous drainage) and sudden hyperemia after surgery.
Intracerebral hematomas reported in the literature are usually ipsilateral to CSH, while our patient developed a remote contralateral occipital bleeding. To our knowledge, remote bleeding after CSH evacuation has been reported in just two other cases68). In a recent paper, Cohen-Gadol6) described a contralateral intraparenchymal hemorrhage associated with intraventricular blood and SAH. The author believes that overdrainage of subdural fluid and CSF was most likely to be the cause of bleeding, since the patient's neurologic conditions improved expeditiously after discontinuation of subdural drainage. It is likely that CSF overdrainage may have led to acute intracranial hypotension and this process placed the contralateral bridging veins under tension, thus causing their collapse and ultimately resulting in venous insufficiency and hemorrhagic infarction.
Dinc et al.8) reported in 2008 the case of a contralateral hematoma after CSH drainage. The authors recommended slow decompression, by twist-drill craniostomy, in order to prevent rapid dynamic intracranial changes.
Our patient developed headache and acute post-operative hypertension and this, in association with CSF drainage and shift of intracranial structures, may have caused the formation of SAH and parenchymal hematoma.
The third patient is an 80-years-old man with a history of hypertension, ischemic heart disease and alcohol abuse. In the post-operative period there was a worsening of neurologic conditions and the head CT showed a contralateral temporal hemorrhage. Unexpectedly, subdural blood along the tentorium and cerebellar hemorrhage within the cerebellar folia were revealed.
The possible pathogenic mechanisms have been illustrated for supratentorial hemorrhages, whereas explanations of remote cerebellar hemorrhage are still tentative. RCH is an infrequent but potentially life-threatening complication that rarely occurs after supratentorial craniotomies and spinal surgery (<5%)2). The incidence of post-operative RCH increased in the CT era. The classic findings comprise subarachnoid blood in the sulci of tentorial surfaces of cerebellar hemispheres and vermis, and additional intracerebellar hemorrhage. In these cases bleeding is characterized by alternating hyperdense (blood), and hypodense (cerebellum) curvilinear and irregular stripes in one or both cerebellar hemispheres. The pattern imitates that of the fur of a zebra, and is thus called zebra sign3). Other depicted CT and magnetic resonance findings are punctuate hemosiderin spots at the superior cerebellar folia, SAH, diffuse hemorrhage in the cerebellar tonsils, vermis and within the fourth ventricle9).
Prolonged awakening from anesthesia, cerebellar symptoms, motor deficits and gait ataxia, diminished consciousness and headache may be signs of RCH. However, RCH may often manifest itself as an asymptomatic incidental finding on post-operative head CT. More severe presentations are related to mass effect upon the fourth ventricle, brainstem compression and development of obstructive hydrocephalus.
The underlying mechanisms of RCH have been extensively discussed in the literature. Currently, most authors agree on multifactorial etiology of RCH2) : CSF overdrainage during or after surgery, episodes of arterial hypertension, impaired venous drainage due to intraoperative head rotation with obstruction of one jugular vein, and coagulation abnormalities have been proposed. In addition, Huang et al.15) recently observed that RCH incidence after supratentorial craniotomy is related with history of cerebrovascular accident with cerebral atrophy. No consensus regarding the exact pathomechanism has been reached. In a wide review and retrospective analysis of 154 cases of RCH, Park et al.26) observed in 2009 the following common features : 1) hemorrhage occurred mostly in the tentorial cerebellar surface, which differed clearly from hypertensive cerebellar hemorrhage, in which dentate nuclei are commonly involved; 2) most cases had SAH with typical streaky bleeding pattern (zebra sign); 3) presentation was incidental in many cases, and in cases with symptomatic deterioration the timing of onset was delayed; 4) drainage systems were used with negative pressure in most cases. On the back of these common features, most authors agree on two facts : 1) RCH is venous in origin, and most likely the superior vermian vein is affected; 2) RCH is likely to be the result of intraoperative and, even more likely, post-operative loss of CSF.
The role of intracranial hypotension has been debated in relation to several post-craniotomy complications31), such as delayed awakening, bradycardia34), brain swelling and RCH. Van Roost et al.32) attributed intracranial hypotension to suction drains. The authors' "mechanistic" negative-pressure theory asserts that the volume removal that occurs when drainage is applied after intracranial surgery may transmit a massive, nonphysiological negative pressure to the brain. This, together with the caudal displacement of the cerebellum due to CSF loss, may induce stretching of cortical veins and venous transmural pressure increase, finally leading to rupture of a small vessel.
While most of RCHs develop after supratentorial craniotomy, only a few cases are reported following burr-hole evacuation of CSH41633). Our patient had arterial hypertension, a history of alcohol consumption, and was assuming antiplatelet therapy prior to surgery. We suppose that rapid brain decompression, in association with an excessive amount of fluid drainage and intracranial hypotension, led to parenchymal shift and consequent vascular damage. This finally resulted in remote hemorrhage in the contralateral temporal lobe and cerebellar hemispheres.
We reported three cases of uncommon hemorrhagic complications after burr-hole craniectomy for CSH. The pathomechanisms are not completely understood. They are probably related to coagulation disorders, arterial and venous pressure imbalance, shift of intracranial contents during surgery, uncontrolled fluid drainage and intracranial hypotension.
Although CSH drainage is considered as a minor procedure, neurosurgeons must be aware of these rare but known adverse events. Correction of coagulopathies, control of blood pressure and management of related medications are essential in order to prevent such complications.
At the time of surgery, careful and gentle evacuation of the subdural hematoma without excessive head rotation is suggested, since delicate irrigation of the cavity of the hematoma is less likely to induce rapid shift of intracranial contents. The use of drains without vacuum bulb is recommended at the end of the operation. Furthermore, tight post-operative monitoring of drainage amounts and characteristics, repeated evaluation of neurologic conditions and prompt radiological assessment are mandatory.
References
1. Arpino L, Gravina M, Basile D, Franco A. Spontaneous chronic subdural hematoma in a young adult. J Neurosurg Sci. 2009; 53:55–57. PMID: 19546844.
2. Brockmann MA, Groden C. Remote cerebellar hemorrhage : a review. Cerebellum. 2006; 5:64–68. PMID: 16527766.
3. Brockmann MA, Nowak G, Reusche E, Russlies M, Petersen D. Zebra sign : cerebellar bleeding pattern characteristic of cerebrospinal fluid loss. Case report. J Neurosurg. 2005; 102:1159–1162. PMID: 16028781.

4. Chang SH, Yang SH, Son BC, Lee SW. Cerebellar hemorrhage after burr hole drainage of supratentorial chronic subdural hematoma. J Korean Neurosurg Soc. 2009; 46:592–595. PMID: 20062580.

5. Cioffi F, Pasqualin A, Cavazzani P, Da Pian R. Subarachnoid haemorrhage of unknown origin : clinical and tomographical aspects. Acta Neurochir (Wien). 1989; 97:31–39. PMID: 2718794.

6. Cohen-Gadol AA. Remote contralateral intraparenchymal hemorrhage after overdrainage of a chronic subdural hematoma. Int J Surg Case Rep. 2013; 4:834–836. PMID: 23959412.

7. de Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CA. Subdural haematoma : a potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry. 2003; 74:752–755. PMID: 12754345.

8. Dinc C, Iplikcioglu AC, Bikmaz K, Navruz Y. Intracerebral haemorrhage occurring at remote site following evacuation of chronic subdural haematoma. Acta Neurochir (Wien). 2008; 150:497–499. discussion 499PMID: 18305890.

9. Dincer A, Özcan Ü, Kaya D, Usseli MI, Erzen C, Pamir MN. Asymptomatic remote cerebellar hemorrhage : CT and MRI findings. Cerebellum. 2012; 11:880–886. PMID: 22249914.

10. Drapkin AJ. Chronic subdural hematoma : pathophysiological basis for treatment. Br J Neurosurg. 1991; 5:467–473. PMID: 1764228.
11. Fogelholm R, Heiskanen O, Waltimo O. Chronic subdural hematoma in adults. Influence of patient's age on symptoms, signs, and thickness of hematoma. J Neurosurg. 1975; 42:43–46. PMID: 1167376.
12. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma : surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005; 107:223–229. PMID: 15823679.

13. Haykowsky MJ, Eves ND, R Warburton DE, Findlay MJ. Resistance exercise, the Valsalva maneuver, and cerebrovascular transmural pressure. Med Sci Sports Exerc. 2003; 35:65–68. PMID: 12544637.

14. Hellwig D, Kuhn TJ, Bauer BL, List-Hellwig E. Endoscopic treatment of septated chronic subdural hematoma. Surg Neurol. 1996; 45:272–277. PMID: 8638225.

15. Huang CY, Lee PH, Lin SH, Chuang MT, Sun YT, Hung YC, et al. Remote cerebellar hemorrhage following supratentorial craniotomy. Neurol Res. 2012; 34:422–429. PMID: 22664148.

16. Hyam JA, Turner J, Peterson D. Cerebellar haemorrhage after repeated burr hole evacuation for chronic subdural haematoma. J Clin Neurosci. 2007; 14:83–86. PMID: 17071089.

17. Infuso L. [Subarachnoid hemorrhage of unknown origin]. Minerva Anestesiol. 1998; 64:149–153. PMID: 9773644.
18. Keller TM, Chappell ET. Spontaneous acute subdural hematoma precipitated by cocaine abuse: case report. Surg Neurol. 1997; 47:12–14. discussion 14-15PMID: 8986158.

19. Kim JK, Kim SW, Kim SH. Intracerebral hemorrhage following evacuation of a chronic subdural hematoma. J Korean Neurosurg Soc. 2013; 53:108–111. PMID: 23560175.

20. Korosue K, Kondoh T, Ishikawa Y, Nagao T, Tamaki N, Matsumoto S. Acute subdural hematoma associated with nontraumatic middle meningeal artery aneurysm : case report. Neurosurgery. 1988; 22:411–413. PMID: 3352892.

21. Missori P, Domenicucci M, Sassun TE, Tarantino R, Peschillo S. Alterations in the intracranial venous sinuses in spontaneous nontraumatic chronic subdural hematomas. J Clin Neurosci. 2013; 20:389–393. PMID: 23219821.

22. Miyazaki T, Matsumoto Y, Ohta F, Daisu M, Moritake K. A case of unknown origin subarachnoid hemorrhage immediately following drainage for chronic subdural hematoma. Kurume Med J. 2004; 51:163–167. PMID: 15373234.

23. Modesti LM, Hodge CJ, Barnwell ML. Intracerebral hematoma after evacuation of chronic extracerebral fluid collections. Neurosurgery. 1982; 10(6 Pt 1):689–693. PMID: 7110541.

24. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases : clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381. PMID: 11561347.

25. Ogasawara K, Ogawa A, Okuguchi T, Kobayashi M, Suzuki M, Yoshimoto T. Postoperative hyperperfusion syndrome in elderly patients with chronic subdural hematoma. Surg Neurol. 2000; 54:155–159. PMID: 11077097.

26. Park JS, Hwang JH, Park J, Hamm IS, Park YM. Remote cerebellar hemorrhage complicated after supratentorial surgery : retrospective study with review of articles. J Korean Neurosurg Soc. 2009; 46:136–143. PMID: 19763216.

27. Sambasivan M. An overview of chronic subdural hematoma : experience with 2300 cases. Surg Neurol. 1997; 47:418–422. PMID: 9131021.
28. Singla A, Jacobsen WP, Yusupov IR, Carter DA. Subdural evacuating port system (SEPS)--minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. 2013; 115:425–431. PMID: 22763191.

29. Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978; 25:651–672. PMID: 710017.

30. Tindall GT, Payne NS 2nd, O'Brien MS. Complications of surgery for subdural hematoma. Clin Neurosurg. 1976; 23:465–482. PMID: 975697.

31. Toledo E, Eynan N, Shalit M. Intracranial hypotension--an iatrogenic complication of vacuum drainage systems. Acta Neurochir (Wien). 1980; 52:55–59. PMID: 7376946.

32. Van Roost D, Thees C, Brenke C, Oppel F, Winkler PA, Schramm J. Pseudohypoxic brain swelling : a newly defined complication after uneventful brain surgery, probably related to suction drainage. Neurosurgery. 2003; 53:1315–1326. discussion 1326-1327PMID: 14633298.
33. Vogels RL, Verstegen MJ, van Furth WR. Cerebellar haemorrhage after non-traumatic evacuation of supratentorial chronic subdural haematoma : report of two cases. Acta Neurochir (Wien). 2006; 148(Wien):993–996. PMID: 16804644.

34. Wasnick JD, Lien CA, Rubin LA, Fraser RA. Unexplained bradycardia during craniotomy closure : the role of intracranial hypotension. Anesth Analg. 1993; 76:432–433. PMID: 7818607.
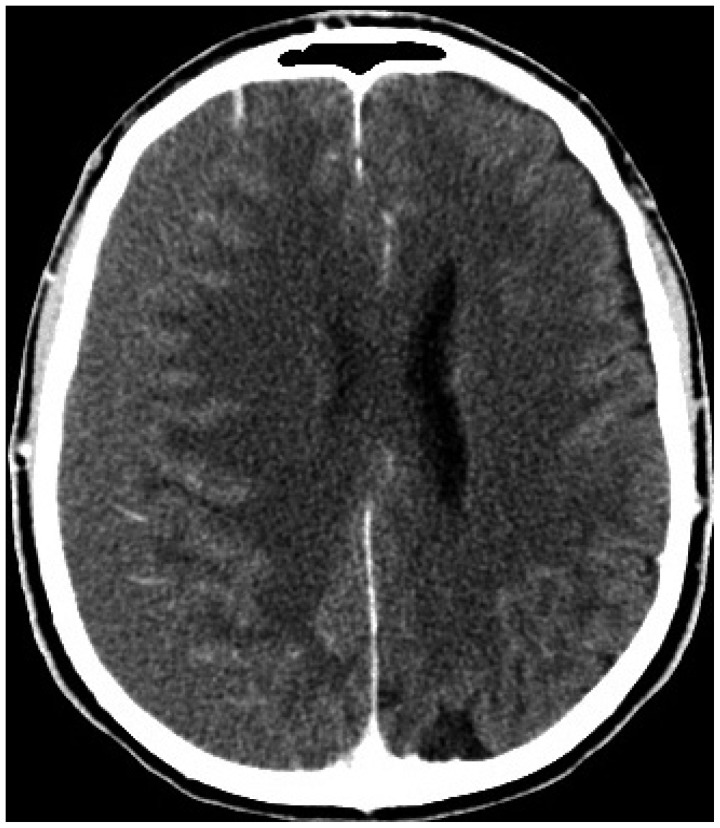
Fig. 2
Post-operative head CT showing diffuse SAH in the basal cisterns (A) and over cerebral convexity (B). SAH : subarachnoid hemorrhage.

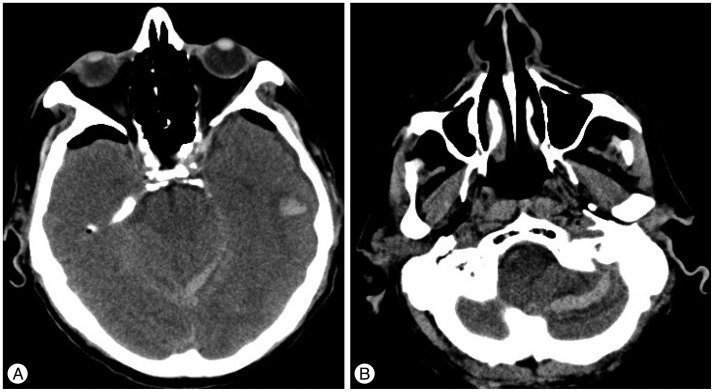
Fig. 4
Right sylvian SAH (A) and contralateral occipital hematoma (B) on a post-operative head CT. SAH : subarachnoid hemorrhage.

Fig. 5
Pre-operative head CT showing a right CSH and a contralateral hemispheric hygroma. CSH : chronic subdural hematoma.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download