Abstract
Methods
A microanatomical study was performed in 79 specimens from 42 formalin-fixed adult cadaver brains. The origins of the ATPAs were divided into anterior, middle, and posterior segments according to the crowding pattern. The morphometry of the ATPAs, including the premammillary artery (PMA), were examined under a surgical microscope.
Results
The anterior and middle segments of the ATPAs arose at mean intervals of 1.75±1.62 mm and 5.86±2.05 mm from the internal carotid artery (ICA), and the interval between these segments was a mean of 3.17±1.64 mm. The posterior segment arose at a mean interval of 2.43±1.46 mm from the posterior cerebral artery (PCA), and the interval between the middle and posterior segments was a mean of 3.45±1.39 mm. The mean numbers of perforators were 2.66±1.19, 3.03±1.84, and 1.67±0.98 in the anterior, middle, and posterior segments, respectively. The PMA originated from the middle segment in 66% of cases. A perforator-free zone was located >2 mm from the ICA in 30.4% and >2 mm from the PCA in 67.1% of cases.
Conclusion
Most perforators arose from the anterior and middle segments, within the anterior two-thirds of the posterior communicating artery (PCoA). The safest perforator-free zone was located closest to the PCA. These anatomical findings may be helpful to verify safety when treating lesions around the PCoA and in the interpeduncular fossa.
The perforating arteries arising from the posterior communicating artery (PCoA) are designated as the anterior thalamoperforating arteries (ATPAs) and number 2-1412151924). Among the perforators, the premammillary artery (PMA) is a very important branch usually defined as the largest and most constant perforating branch of the PCoA penetrating the area between the optic tract, the mammillary bodies, and the cerebral peduncle so-called paramedian perforating substance1211121315192024). The PCoA has a deep and narrow course that increases the risk of injury to the PCoA perforating vessels during vascular or tumor surgery in the parasellar region, mesial temporal lobe, or interpeduncular fossa. These perforating vessels are usually end arteries; thus, injury produces neurologic sequela. Thus, it is crucial to preserve them during surgery, so extensive anatomical knowledge is needed. The purpose of this study was to evaluate the morphometric characteristics of the ATPAs.
Forty-two (27 male; mean age, 60.4 years; range, 23-83 years and 15 female; mean age, 54.3 years; range, 28-86 years) formalin-fixed human cadaveric brains were used. The brains were removed carefully from the skull to avoid damage to the brain stem and to preserve the internal carotid artery (ICA) proximal to the PCoA. The brain was placed upside down, and the ICA, PCoA, anterior choroidal artery (AchoA), and posterior cerebral artery (PCA) were examined on the ventral surface of the brain stem. The perforators arising from the junction of the PCoA and the ICA (IC-PC junction) to the PCA were dissected bilaterally under a surgical microscope (Fig. 1). Among the 84 bilateral specimens from the 42 cadaveric brains, four specimens could not be examined due to severe damage to the brain stem and its vasculature. Of the 80 specimens, the AchoA and PCoA had a common origin in two specimens (2.5%) and the PCoA was absent in one specimen (1.3%) (Fig. 2). Because of the absence of the PCoA in one specimen, morphometric measurements were taken from 79 specimens (52 males and 27 females) using digital calipers (Mitutoyo Co., Tokyo, Japan) after spreading the folded PCoA. Among 79 specimens, fetal-type PCoA was seen in seven specimens (8.9%). The PMA was defined as the largest perforating vessel penetrating between the optic tract, the mammillary bodies, and the cerebral peduncle (Fig. 3).
According to the crowding pattern of perforators arising from the PCoA, the ATPAs were roughly divided into anterior, middle, and posterior segments. The measurement taken in this study were as follows : 1) length and diameter of the PCoA; 2) interval and length of each segment; 3) number of perforators and the largest diameter perforators in each segment; 4) origin and the largest diameter of the PMA; 5) distribution of the perforator-free zone (PFZ) from the ICA and PCA. Schematic and cadaveric views of the anatomical measurements are shown in Fig. 4. Student t-test was used to compare mean differences between right and left sides. A p-value <0.05 was considered significant.
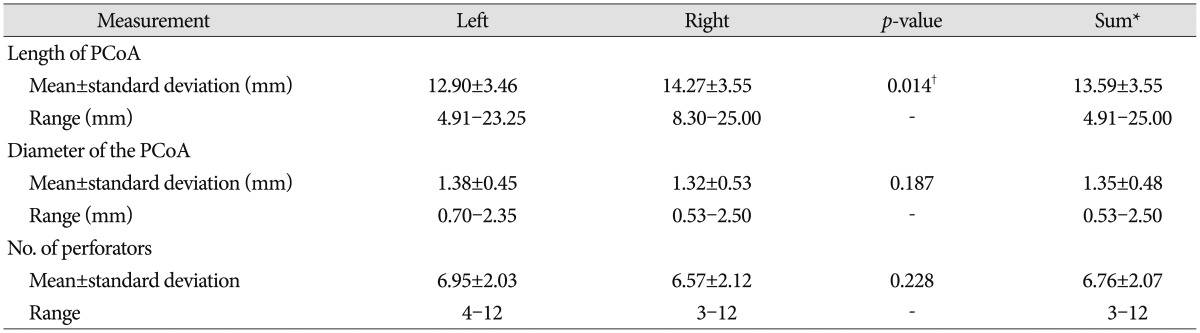
The mean length and diameter of the PCoA were 13.59±3.55 mm (range, 4.91-25.00 mm) and 1.35±0.48 mm (range, 0.53-2.50 mm), respectively. The right PCoA was significantly longer than the left PCoA (p=0.014), and mean diameter was 1.32 mm on the right and 1.38 on the left. The length and diameter of the PCoA varied widely. The mean number of perforators was 6.76±2.07 (range, 3-12; 6.95 for the left and 6.57 for the right PCoA) (Table 1).
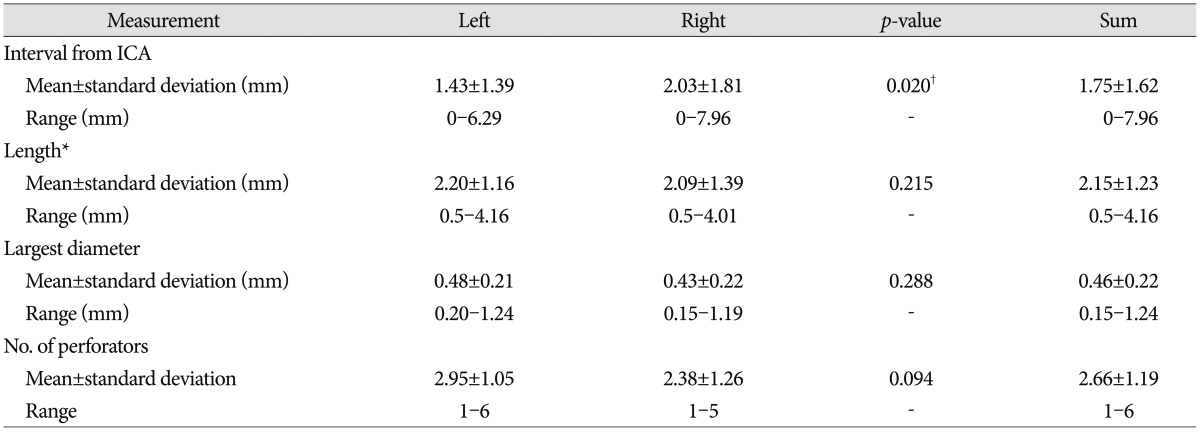
The anterior segment of the ATPAs arose at a mean interval of 1.75±1.62 mm (range, 0-7.96 mm) from the ICA, with an average length of 2.15±1.23 mm (range, 0.5-4.16 mm). The interval from the ICA to the so-called PFZ from the ICA was slightly but significantly longer on the right than that on the left (p=0.020), which may have corresponded with PCoA length. The first perforator arose 2 mm from the ICA in 55 specimens (69.6%) and >2 mm from the ICA in the remaining 24 specimens (30.4%). The mean diameter of the largest perforator was 0.46±0.22 mm (range, 0.15-1.24 mm), and the mean number of perforators was 2.66±1.19 (range, 1-6). No significant differences were observed in length, diameter, or the number of perforators between the right and left sides (Table 2). Perforators emerged from the IC-PC junction in one specimen.
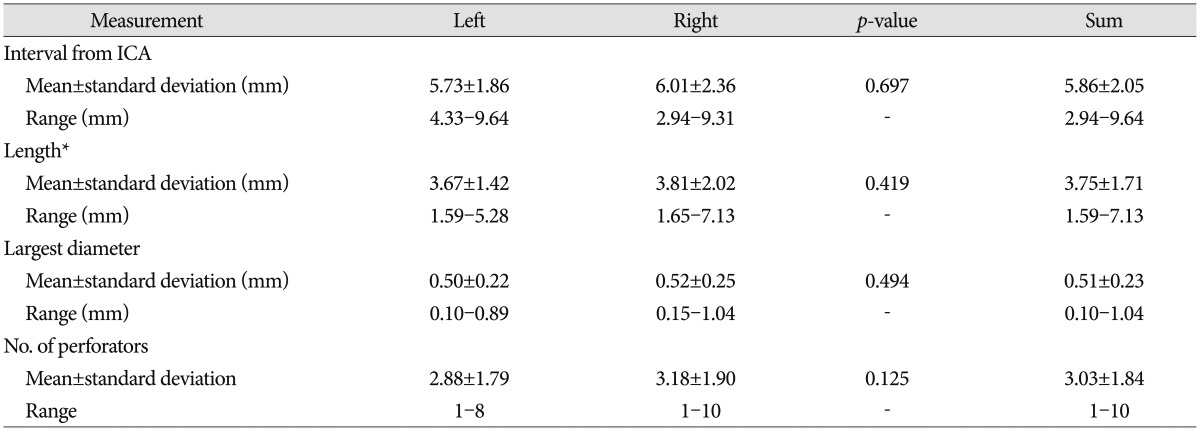
The middle segment of the ATPAs arose at a mean interval of 5.86±2.05 mm (range, 2.94-9.64 mm) from the ICA and 3.17±1.64 mm (range, 1.20-7.10 mm) from the anterior segment, with a mean length of 3.75±1.71 mm (range, 1.59-7.13 mm). The mean diameter of the largest perforator was 0.51±0.23 mm (range, 0.10-1.04 mm), and the mean number of perforators was 3.03±1.84 (range, 1-10) (Table 3). The middle segment occupied the largest area of the PCoA, and the perforators had the largest diameters and were the most numerous among the three ATPA segments. Both the anterior and middle segments occupied roughly the anterior two-thirds of the PCoA (Fig. 4).
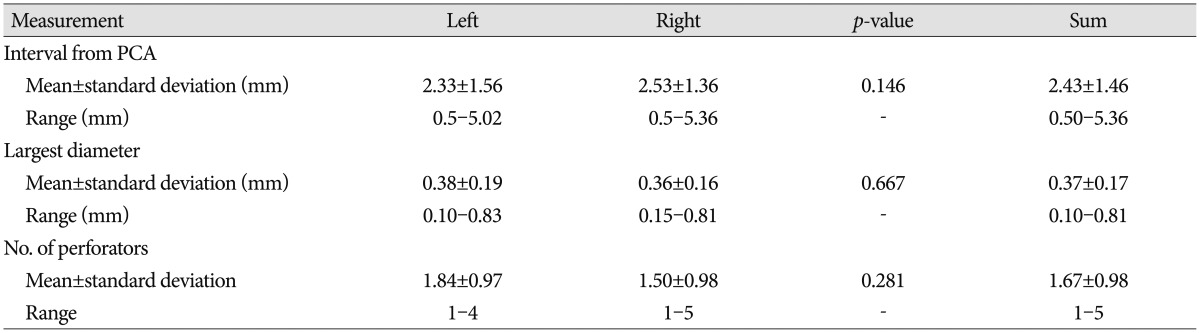
The posterior segment of the ATPAs arose at a mean interval of 2.43±1.46 mm from the PCA (range, 0.50-5.36 mm) and 3.45±1.39 mm from the middle segment. The perforators arose ≤2 mm from the PCA in 26 specimens (32.9%) and >2 mm from the PCA in the remaining 53 specimens (67.1%). The mean number of perforators was 1.67, and a very fine single perforator was observed in 48 (60.8%) of 79 specimens (Fig. 4). The mean diameter of the largest perforators was 0.37±0.17 mm (range, 0.10-0.81) (Table 4).
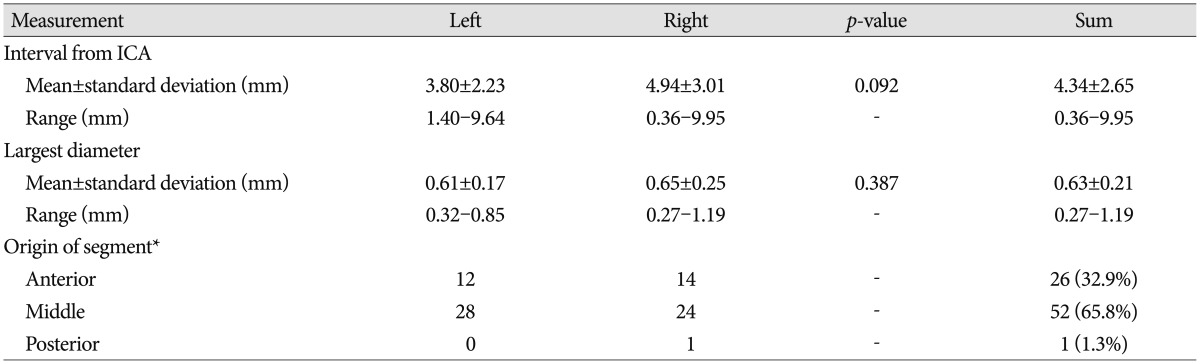
The PMA arose at a mean interval of 4.34±2.65 mm (range, 0.36-9.95 mm) from the ICA, and mean diameter was 0.63±0.21 mm (range, 0.27-1.19 mm) (Table 5). The PMA originated from the middle segment in 65.8% and from the anterior segment in 32.9% of cases. However, some variations were noted. The PMA was formed by an anastomosis with a perforator from the PCA and a perforator from the posterior segment of the PCoA in one specimen, and a double PMA was seen in one specimen which formed in 0.01% of the cases (Fig. 2). And anastomoses between perforators were found in three specimens (Fig. 2B, 5). The PMA originated from the medial, superior, and superolateral walls of the PCoA with wide variation but usually from the superior surface of the PCoA (Fig. 6). No correlation was found between PCoA diameter and the number, diameter, or originating patterns of the perforators, including the PMA. PMA diameter in one specimen was almost the same as that of the parent PCoA. A reduction in PCoA diameter after emergence of the PMA was seen in two (2.5%) of 79 specimens (Fig. 7).
The intervals between each segment and the distribution of the PFZ are demonstrated schematically in Fig. 4. Of the 79 specimens, 67.1% had a PFZ >2 mm from the PCA (mean, 2.43 mm), whereas 30.4% had a PFZ >2 mm from the ICA (mean, 1.75 mm). The mean gaps between each ATPA segment were 3.17±1.64 (range, 1.20-7.44 mm) and 3.45±1.39 mm (range, 0.87-6.24 mm) between the anterior and middle segments and between the middle and posterior segments, respectively (Table 6).
The PCoA arises from the posteromedial, the posterior, or the posterolateral aspect of the ICA and passes posteromedially to join the PCA. It passes through the carotid cistern and pierces Liliequist's membrane to enter the interpeduncular cistern where it joins the PCA and numerous perforating branches arising from the PCoA are found21224). This relatively deep and narrow course of the PCoA with complex perforating branch anatomy increases the risk of injury to the PCoA perforating vessels during vascular or tumor surgery in the parasellar region, mesial temporal lobe, or interpeduncular cistern. Umredkar et al.27) reported a 35.71% rate of radiologically proven perforator-related infarctions after PCoA aneurysm clipping, however including incidence of infarction by vasospasm. These perforating vessels are usually end arteries; thus, injury will produce neurologic sequela, so it is crucial to preserve them during surgery, and extensive anatomical knowledge is needed. In endovascular surgery, thromboembolic events are a fearful complication to be avoided reported to be 2.5-28% of patients treated in cases of aneurysmal subarachnoid hemorrhage6212629). Endo et al.9) reported thromboembolic complications in PCoA perforator territory during coil embolization and found a difference in infarction size depending on PMA involvement or not, as the PMA has an anatomically complementary relationship with the paramedian artery originating from the P1 segment, which emphasizes the importance of the PMA.
According to our morphological data, the PCoA was slightly but significantly longer on the right side than that on the left. The distance between the ICA and the first PCoA perforator in the anterior segment was slightly but significantly longer in the right PCoA than that in the left PCoA. This result can be helpful to determine the side to approach the interpeduncular fossa when there is no better approach based on the lesion, and an angiogram does not show the PCoA well enough to determine which side would be a better approach. As the side with the longer PCoA creates a wider operative field in the interpeduncular fossa, the right side can be favorable in such a situation.
The PCoA has been divided into various types depending on the degree of involution, which is based on PCoA embryology28). When the diameter of the PCoA exceeds the diameter of the P1, it is called fetal type, and when equal, transitional type, when the diameter is less than 1mm, it is called hypoplastic type. And when the diameter exceeds 1 mm but less than the diameter of the P1, it is called adult type. The incidences of the fetal, transitional, and hypoplastic types are 15-40%, 8-18%, and 18-32%, respectively142428). In our study, the fetal type PCoA was found in 8.9% of the 79 specimens, which is very low compared to the literature. However, no correlation was found between PCoA type and the diameters or patterns of the branching perforators. Similar findings with other previous reports, the type of PCoA does not make any difference on the importance of the perforator1291415192330). In a specimen, the diameter of the PMA was almost the same as that of the parent hypoplastic PCoA, which supports this finding and ATPAs greater in diameter than the parent PCoA have been reported2).
Interventional treatments are continually developing. Open surgery has been replaced for posterior circulation aneurysm treatment. However, there are some limitations based on the morphology of the aneurysm and the parent artery. A wide-necked PCoA aneurysm occasionally incorporates the PCoA at its origin and obstruction of the PCoA is thus considered a therapeutic strategy59). The Allcock test is useful to evaluate collateral blood flow and predict postoperative ischemic complications encountered after sacrificing the PCoA. However, Endo et al.9) reported infarctions in perforator territory after occluding the PCoA, even with a positive Allcock test result, suggesting that although an angiogram shows filling of the PCoA by the PCA, it does not meet the physiological demand of circulation. We found two specimens (2.5%) with reduced PCoA diameter after emergence of the PMA (Fig. 7). Gabrovsky11) found up to 20% reduced PCoA diameter from its anterior to its posterior third in 27% of adult type PCoAs and 24% of hypoplastic type PCoAs, which supports our finding, and explains the insufficient retrograde flow from the PCA to PCoA in such case. Therefore, occlusion of the PCoA should be done with great care, even with a positive Allcock test result.
According to previous studies, the average number of ATPAs is 4-1412151924). These perforators originate from the superior aspect of the PCoA, either medially or laterally, with wide variations (Fig. 6). ATPAs have a variable initial course but finally run posteromedially into the interpeduncular cistern and supply the inferior optic chiasm, optic tract, tuber cinereum, mammillary bodies, subthalamus, posterior hypothalamus, and anterior thalamus (Fig. 1). Damage to these perforators can lead to amnesia, subcortical cognitive disturbances, endocrine dysfunction, visual field defects, vegetative disorders, hyperthermia, motor weakness, and sensory and personality changes23111415181924).
In this study, we determined that the perforators had a specific branching pattern. They arose in three different crowding patterns, and we divided them into three segments of anterior, middle, and posterior according to the crowding pattern (Fig. 4). The middle segment occupied the largest area of the PCoA (mean length, 3.75±1.71 mm), and the perforator had the largest diameter (mean diameter of the largest perforator, 0.51±0.23 mm) and was the most numerous among the three ATPA segments (mean number of perforators, 3.03±1.84). The fewest number of perforators and those with the smallest diameter were found in the posterior segment (mean number of perforators, 1.67±0.98; mean diameter of the largest perforator, 0.37±0.17 mm), and only a single fine perforator was found in 60.8% of the posterior segments. Similar to previous studies13111214151624), most perforators arose from the anterior and middle segments, which roughly occupied the anterior two-thirds of the PCoA (Fig. 4).
The ATPAs course in a group by their segment. Similar findings were reported by Vincentelli et al.28). In their study, they divided ATPAs into medial and lateral groups based on the direction of the perforators and reported that they were enclosed within their own arachnoid compartment. They stated that lifting the perforators together in a group with their arachnoid layers makes it easier to pass the perforators to the aneurysm when operating on a basilar tip aneurysm. According to our finding that the perforators were grouped by segments, this can be a tip to predict perforator location and preserve them when performing PCoA aneurismal clipping. For example, when clipping a large PCoA aneurysm with a wide neck, the operation field may not be wide enough to expose the perforators satisfactorily. Instead of suffering to find all perforators individually, just identifying the group of perforators by their segment in their arachnoid layers which can be more simple, we can predict the perforator free area and attempt clipping with confidence that the perforators will be safe. Although, of course, all efforts should be made for inspection of perforators in detail after clipping since there are variable findings of perforators other than the usual pattern reported in our study, such as perforators emerging from the junction of PCoA and ICA and so on.
The largest and most constant PCoA perforator is referred to as the PMA (Fig. 3)12111213192024). In historical perspective, there has been inconclusive nomenclauture of the perforators including the PMA. Duret78) reported perforating arteries as anterior internal optic arteries in 1874, and they were referred to as the premammillary or thalamotuberal pedicle by Foix and Hillemand10) in 1925. Later, the largest and longest branch of the PCoA was called the thalamic polar artery by Lazorthes and Salamon17). Zeal and Rhoton31) reported the perforating branches as anterior thalamoperforating arteries and the largest of them as the premammillary artery in 1978. We used nomenclature in which the PMA is referred to as the largest and most constant branch penetrating the paramedian perforating substance, which is the area surrounded by the mammillary bodies, optic tract, and the cerebral peduncle first described by Percheron (Fig. 3)22). The PMA supplies the posterior hypothalamus, mammillothalamic tract, anterior thalamus (nucleus ventralis anterior, ventrolateral and dorsomedial nucleus, and reticular nucleus), anteromedial part of the optic tract, inferomedial tip of the head of the caudate nucleus, the genu, and part of the posterior limb of the internal capsule1112132024). Occlusion of the PMA has been described as a clinical syndrome, including contralateral motor weakness, changes in superficial modalities of sensation, neuropsychological dysfunction, such as apathy, lack of spontaneity, disorientation, and memory and language disturbances111213).
Gibo et al.13) reported that the usual site of emergence of the PMA is the caudal third of the PCoA, which was opposite to our result. We found the PMA located at a mean interval of 4.34 mm from the ICA. It arose from the middle segment of the PCoA in 66% of cases and mostly within the anterior two-thirds of the PCoA. In the microanatomical study with 70 cadaveric hemispheres by Gabrovsky et al.12), PMA was found to originate from the middle third of the PCoA in 50% of the cases, from the anterior third in 17%, similar findings with our study.
The PMA is usually considered a single branch of the PCoA, but it can be plural. Duplication of the PMA was observed in 2% of cases by Pedroza et al.1920) and in 28.8% of cases by Gibo et al.13). Triplication of the PMA has also been documented in 4.3% of cases by Gabrovsky et al.12). We found a duplicate PMA in one case (0.01% of cases) (Fig. 2).
In view point of the location and morphology of perforators, the safest PFZ can be considered to be typically located closest to the PCA, corresponding with previous reports that most ATPAs, including the most important perforator, the PMA, arises from the anterior two-thirds of the PCoA13111214151624). This finding is important when operating on the interpeduncular fossa. In a case when the PCoA is short, it can act like a tension band between the ICA and PCA, making it difficult to widen the operating window and occasionally the PCoA perforator can hinder the lesion, so in such case dividing the PCoA is necessary. Many reports have described safe division of the caudal PCoA and preserving the perforators due to the fewer number of perforators located in the posterior third of the PCoA311121314151623242530). Beumer et al.3) reported that the length of the PFZ is 2-6 mm and that the longest PFZ is located closer to the PCA than to the ICA. This was similar to our finding. The mean distance between the site of the first perforator and the ICA was 1.8 mm in their study, and that between the last perforator and the PCA was 2.4 mm. The PFZ was >2 mm from the PCA in 67% of specimens, whereas 70% of cases had a PFZ within 2 mm from the ICA (Table 6). However, we found a specimen with a PMA formed by an anastomosis between a perforator from the PCA and the posterior segment of PCoA (Fig. 2). In such case, dividing the caual part of PCoA can be dangerous.
Although many studies have reported measurements and categorized the PCoA and its perforators, a large number of variations exist. As examples, one specimen had a common origin for the AchoA and PCoA, another had a PMA formed by an anastomosis of a PCA perforator and a perforator of the posterior segment of the PCoA, and a specimen of a double PMA was found (Fig. 2). Perforators emerging from the junction of the PCoA and ICA were also seen. Other studies have reported PMA variants. In a study by Gabrovsky et al.12), a PMA originating from the P1 segment of the posterior cerebral artery or ICA was found and other studies reported double, triple PMA as described above131920). As mentioned earlier, atypical from our study, emergence of PMA from the caudal PCoA was reported13). Regarding these variations, the results described in this study should just be a reference, and a detailed microscopic inspection of the PCoA and it's perforators should be carried out on operation.
The PCoA perforators arise from anterior, middle, and posterior segments of the PCoA according to their crowding pattern. Most perforators arise from the anterior and middle segments, which are located within the anterior two-thirds of PCoA. The PFZ was located >2 mm from the PCA in 67% of cases and within 2 mm from the ICA in 70% of cases. The PMA was located at a mean interval >4 mm from the ICA within the anterior two-thirds of the PCoA and mostly in the middle segment of the ATPA. Considering the location and morphology of the perforators, the safest PFZ was typically located closest to the PCA. These anatomical findings may be helpful to verify safety when performing surgical clipping or endovascular coiling procedures on the PCoA, basilar artery aneurysms, or tumor surgery in the interpeduncular fossa. However, there are a large number of variations, and results of this study should just be a reference, and a detailed microscopic inspection of the PCoA and it's perforators should be carried out on operation.
References
1. Avci E, Bademci G, Oztürk A. Posterior communicating artery : from microsurgical, endoscopic and radiological perspective. Minim Invasive Neurosurg. 2005; 48:218–223. PMID: 16172967.

2. Baskaya MK, Coscarella E, Gomez F, Morcos JJ. Surgical and angiographic anatomy of the posterior communicating and anterior choroidal arteries. Neuroanatomy. 2004; 3:38–42.
3. Beumer D, Delwel EJ, Kleinrensink GJ, Akouri S, Torres A, Krisht AF. The perforator-free zone of the posterior communicating artery and its relevance in approaches to the interpeduncular cistern, especially the transcavernous approach : an anatomic study. Neurosurgery. 2007; 61(5 Suppl 2):187–191. discussion 191-192PMID: 18091232.
4. Bisaria KK. Anomalies of the posterior communicating artery and their potential clinical significance. J Neurosurg. 1984; 60:572–576. PMID: 6699700.

5. Cho YD, Jung SC, Kim CH, Ahn JH, Kang HS, Kim JE, et al. Posterior Communicating Artery Compromise in Coil Embolization of Posterior Communicating Artery Aneurysms. Clin Neuroradiol. 2014; [Epub ahead of print]. DOI: 10.1007/s00062-014-0308-4.

6. Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils : the preliminary University of Illinois at Chicago experience. Neurosurgery. 1998; 43:1281–1295. discussion 1296-1297PMID: 9848841.

7. Duret H. Recherches anatomiques sur la circulation de l'encéphale. Arch Physiol Norm Pathol. 1874; 1:60–91.
8. Duret H. Recherches anatomiques sur la circulation de l'encéphale. Arch Physiol Norm Pathol. 1874; 2:919–957.
9. Endo H, Sato K, Kondo R, Matsumoto Y, Takahashi A, Tominaga T. Tuberothalamic artery infarctions following coil embolization of ruptured posterior communicating artery aneurysms with posterior communicating artery sacrifice. AJNR Am J Neuroradiol. 2012; 33:500–506. PMID: 22194388.

10. Foix CH, Hillemand P. Les arteres de l'axe encephalique jusqu'au diencephale inclusivement. Rev Neurol. 1925; 2:705–739.
11. Gabrovsky N. Microanatomical bases for intraoperative division of the posterior communicating artery. Acta Neurochir (Wien). 2002; 144:1205–1211. PMID: 12434177.

12. Gabrovsky S, Laleva M, Gabrovsky N. The premamillary artery--a microanatomical study. Acta Neurochir (Wien). 2010; 152:2183–2189. PMID: 20700746.

13. Gibo H, Marinkovic S, Brigante L. The microsurgical anatomy of the premamillary artery. J Clin Neurosci. 2001; 8:256–260. PMID: 11386802.

14. Inao S, Kuchiwaki H, Hirai N, Gonda T, Furuse M. Posterior communicating artery section during surgery for basilar tip aneurysm. Acta Neurochir (Wien). 1996; 138:853–861. PMID: 8869714.

15. Krayenbühl N, Krisht AF. Dividing the posterior communicating artery in approaches to the interpeduncular fossa : technical aspects and safety. Neurosurgery. 2007; 61(5 Suppl 2):392–396. discussion 396-397PMID: 18091254.
16. Krisht AF, Kadri PA. Surgical clipping of complex basilar apex aneurysms : a strategy for successful outcome using the pretemporal transzygomatic transcavernous approach. Neurosurgery. 2005; 56(2 Suppl):261–273. discussion 261-273PMID: 15794823.
17. Lazorthes G, Salamon G. The arteries of the thalamus : an anatomical and radiological study. J Neurosurg. 1971; 34:23–26. PMID: 5539644.
18. Lisovoski F, Koskas P, Dubard T, Dessarts I, Dehen H, Cambier J. Left tuberothalamic artery territory infarction : neuropsychological and MRI features. Eur Neurol. 1993; 33:181–184. PMID: 8467830.

19. Pedroza A, Dujovny M, Artero JC, Umansky F, Berman SK, Diaz FG, et al. Microanatomy of the posterior communicating artery. Neurosurgery. 1987; 20:228–235. PMID: 3561728.

20. Pedroza A, Dujovny M, Cabezudo-Artero J, Umansky F, Berman SK, Diaz FG, et al. Microanatomy of the premamillary artery. Acta Neurochir (Wien). 1987; 86:50–55. PMID: 3618306.

21. Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1998; 19:1541–1547. PMID: 9763391.
22. Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol. 1973; 205:1–13. PMID: 4126735.

23. Regli L, de Tribolet N. Tuberothalamic infarct after division of a hypoplastic posterior communicating artery for clipping of a basilar tip aneurysm : case report. Neurosurgery. 1991; 28:456–459. PMID: 2011233.

24. Saeki N, Rhoton AL Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. 1977; 46:563–578. PMID: 845644.

25. Sugita K, Kobayashi S, Shintani A, Mutsuga N. Microneurosurgery for aneurysms of the basilar artery. J Neurosurg. 1979; 51:615–620. PMID: 501400.

26. Tummala RP, Chu RM, Madison MT, Myers M, Tubman D, Nussbaum ES. Outcomes after aneurysm rupture during endovascular coil embolization. Neurosurgery. 2001; 49:1059–1066. discussion 1066-1067PMID: 11846898.

27. Umredkar A, Gupta SK, Khandelwal N, Chhabra R, Mathuriya SN, Pathak A, et al. Intracerebral infarcts following clipping of intracranial aneurysms : incidence, clinical correlation and outcome. Br J Neurosurg. 2010; 24:156–162. PMID: 20210531.

28. Vincentelli F, Caruso G, Grisoli F, Rabehanta P, Andriamamonjy C, Gouaze A. Microsurgical anatomy of the cisternal course of the perforating branches of the posterior communicating artery. Neurosurgery. 1990; 26:824–831. PMID: 2352600.

29. Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm : perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997; 86:475–482. PMID: 9046305.

30. Yasargil MG, Antic J, Laciga R, Jain KK, Hodosh RM, Smith RD. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg Neurol. 1976; 6:83–91. PMID: 951657.
31. Zeal AA, Rhoton AL Jr. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg. 1978; 48:534–559. PMID: 632878.

Fig. 1
Photographs showing the posterior communicating artery, perforators and relative anatomical structures on infero-superior view of cadaveric specimen. A : optic chiasm, B : internal carotid artery, C : posterior communicating artery, D : optic tract, E : anterior thalamoperforating arteries, F : cerebral peduncle, G : P2 segment of posterior cerebral artery, H : P1 segment of posterior cerebral artery, I : mammillary body, J : tuber cinereum, K : anterior choroidal artery.

Fig. 2
Phtographs showing the variants of the posterior communicating artery (PCoA) and premammillary artery (PMA). A : The PMA (black arrow) is formed by an anastomosis of a perforator from the posterior cerebral artery (PCA) (black star) and a perforator from the posterior segment of the PCoA (black arrowhead). B : A specimen showing a double PMA (black arrowheads) and an anastomosis (black star) between perforators from the middle segment of PCoA (black arrows). C : A specimen showing absence of PCoA (white circle) on infero-superior view. D : A specimen showing a common origin of the anterior choroidal artery (AchoA) and the PCoA indicated by the black star. Enlarged view of the common origin of the AchoA and PCoA is shown in the inner box. BA : basilar artery, I : infundibulum, ICA : internal carotid artery, MB : mammillary body, OC : optic chiasm, SCA : superior cerebellar artery, TC : tuber cinereum.

Fig. 3
Photograph showing the paramedian perforating substance on infero-superior view of cadaveric specimen. Premammillary artery (black arrowhead) and anterior thalamoperforating arteries penetrate the paramedian perforating substance which is surrounded by the mammillary bodies, optic tract, and the cerebral peduncle, indicated by the white triangle. A : optic tract, B : posterior communicating artery, C : internal carotid artery, D : cerebral peduncle, E : posterior cerebral artery, F : mammillary body.

Fig. 4
Schematic view and cadaveric specimen showing the segments of posterior communicating artery (PCoA) according to the crowding pattern of anterior thalamoperforating arteries. Black arrows mean the length of each segment occupying the PCoA. Red arrows indicate the interval between each segment. Red circles indicate the perforator-free zone from the internal carotid (ICA) and the posterior cerebral arteries (PCA), respectively. Fine single perforator arising from the posterior segment of PCoA is visible. Both anterior and middle segments roughly occupied the anterior two-thirds of the PCoA. Ant : anterior segment, Mid : middle segment, Post : posterior segment.
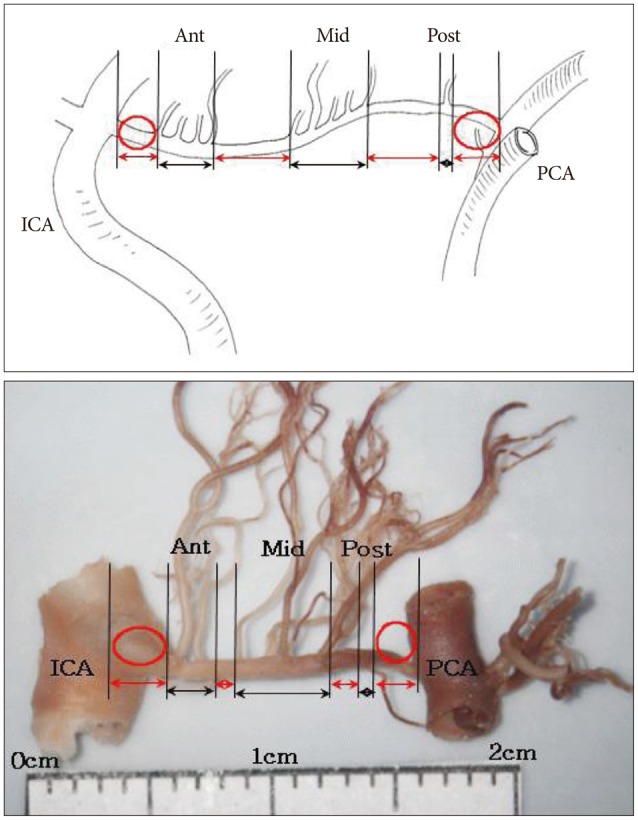
Fig. 5
Photographs showing the anastomoses between perforators. A : Anastomosis between premammillary artery (black arrowhead) and a perforator from the posterior segment of posterior communicating artery (PCoA) (black arrow). B : Anastomsis between the perforator from the posterior segment of PCoA (black arrowhead) and a perforator from the PCA (black arrows). Black star indicates the anastomosis site. Each segment of PCoA is indicated. ICA : internal carotid artery, PCA : posterior cerebral artery.
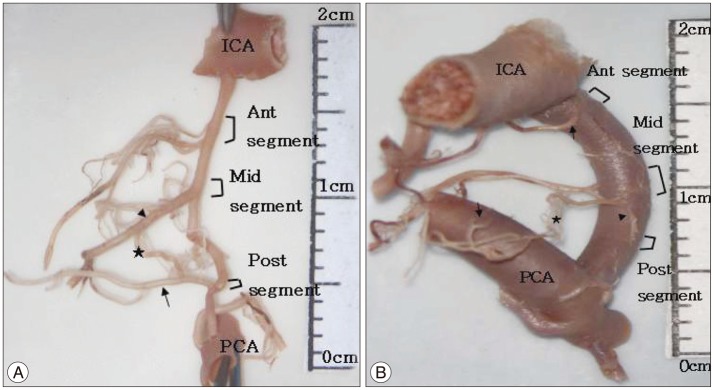
Fig. 6
Cadaveric specimen showing the origin surface of premammillary artery (PMA) (black arrowhead) arising from posterior communicating artery (PCoA). A : PMA originates from the superolateral surface of the right PCoA. B : PMA originates from the lateral surface of the left PCoA. C : PMA originates from the medial surface of the right PCoA, ICA : internal carotid artery.
Fig. 7
Cadaveric specimens showing the reduction of diameter of the posterior communicating artery (PCoA) after the emergence of the premammillary artery (PMA). Arrowhead indicates PMA, blackstar indicates the reduction of diameter of the PCoA, and black arrow indicates single fine perforator arising from posterior segment of PCoA. ICA : internal carotid artery, PCA : posterior cerebral artery.
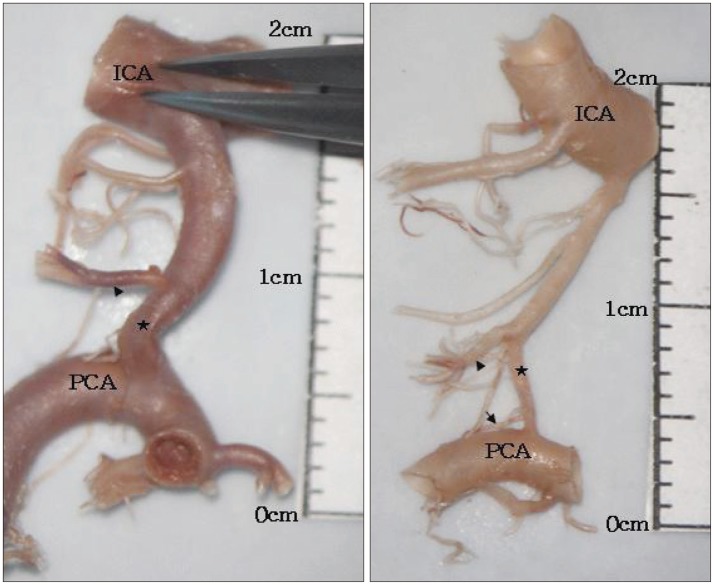
Table 6
Morphometric aspects of the interval between each segment and the PFZ
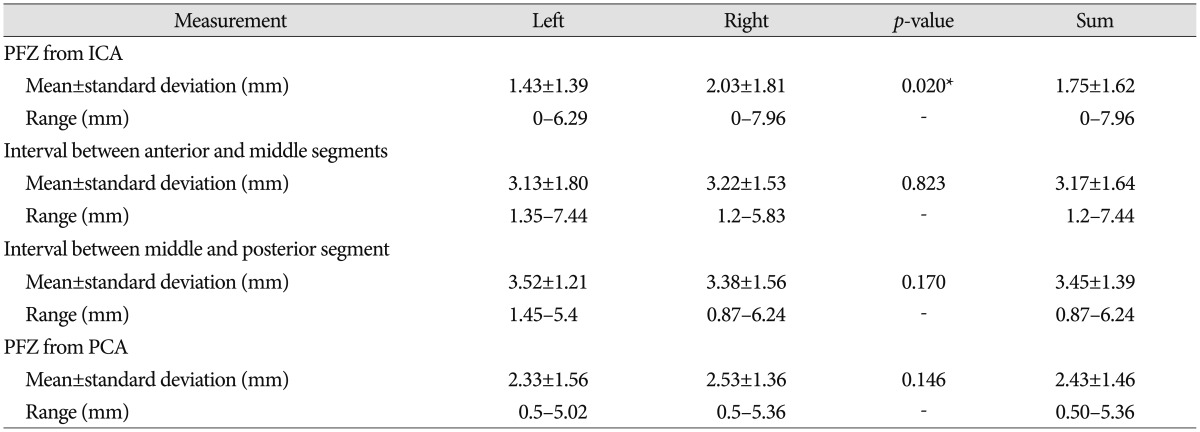
ant seg : anterior segment of the anterior thalamoperforating artery (ATPA), mid seg : middle segment of the ATPA, post seg : posterior segment of the ATPA. *Significant difference between left and right sides. ICA : internal carotid artery, PCA : posterior cerebral artery, PFZ : perforator-free zone




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download