Abstract
Objective
Malignant gliomas are the most common primary tumors of the central nervous system and the prognosis of patients with gliomas is poor. The combination of interferon-bata (IFN-β) and temozolomide (TMZ) has shown significant additive antitumor effects in human glioma xenograft models. Considering that the poor survival of patients with human malignant gliomas relates partly to the inability to deliver therapeutic agents to the tumor, the tropism of human bone marrow-derived mesenchymal stem cells (MSC) for malignant gliomas can be exploited to therapeutic advantages. We investigated the combination effects of TMZ and MSCs that secrete IFN-β on gliomas.
Methods
We engineered human MSCs to secret mouse IFN-β (MSC-IFN-β) via adenoviral transduction and confirmed their secretory capacity using enzyme-linked immunosorbent assays. In vitro and in vivo experiments were performed to determine the effects of the combined TMZ and MSC-IFN-β treatment.
Glioblastoma (GBM) is one of the most common primary brain tumors in the central nervous system in adults, accounting for approximately 40% of all brain tumors5). Currently, temozolomide (TMZ)-based chemotherapy with concomitant radiotherapy is accepted as the first line therapy for malignant gliomas10). However, despite aggressive treatment, most patients with gliomas have a median survival time of about one year after diagnosis. The unsatisfactory response to TMZ treatment is related to the DNA repair protein, O6-methylguanine-DNA methyltransferase (MGMT). Accordingly, many studies have reported the use of TMZ in combination with other therapies to modify the expression of MGMT, including histone deacetylase inhibitors such as vorinostat and valproic acid9). Additionally, interferon-beta (IFN-β) has been reported in a phase I clinical trial in combination with TMZ11).
IFN-β is known to act as a drug sensitizer and is widely used in combination with other antitumor agents7). The combination of IFN-β and TMZ has shown significant synergistic antitumor effects in human glioma xenograft models. IFN-β is known to inactivate the expression of MGMT via p53 gene induction, thus it enhances the therapeutic efficacy of TMZ8). The results of the clinical trial using the combined treatment of IFN-β and TMZ showed that the median overall survival of the combination group was significantly greater (19.9 months) compared to the TMZ alone group (12.7 months) by about 7 months11).
Although the current study also utilizes the combined treatment of IFN-β and TMZ, our study is unique in that we focused on delivering the therapeutic agents to the tumor. Specifically, we used mesenchymal stem cells (MSCs) as a porter. MSCs have a pathotropism for human gliomas after intravascular or local delivery6). Therefore, considering that the poor survival of patients with human malignant gliomas is related, at least in part, to the inability to deliver therapeutic agents to the tumor, the tropism of human MSCs for malignant gliomas can be exploited for therapeutic purposes, i.e., MSCs can be used as delivery vehicles with tumor-targeting capabilities.
In the present study, we engineered human MSCs to secret mouse IFN-β (MSC-IFN-β) and investigated the combination effects of MSC-IFN-β and TMZ using in vitro and in vivo experiments.
Human bone marrow-derived MSCs (Catholic MASTER Cells) were obtained from the Catholic Institute of Cell Therapy (Seoul, Korea). Cells were thawed, and the culture process was performed according to the manufacturer's instructions. Human bone marrow aspirates were obtained from the iliac crest of healthy donors aged 20 to 55 years after approval by the Institutional Review Board of Seoul St. Mary's Hospital (approval numbers KIRB-00344-009 and KIRB-00362-006). Bone marrow aspirates from each consented donor were sent to the GMP-compliant facility of the Catholic Institute of Cell Therapy (Seoul, Korea, http://www.cic.re.kr) for the isolation, expansion, and quality control of human bone marrow-derived MSCs. Cells were plated in a culture dish and cultured with human bone marrow-derived MSC basal medium supplemented with MSC growth supplement at 37℃ with 5% CO2. The MSC growth medium was used for all cell expansion procedures, unless otherwise mentioned. During cell expansion, cells were tested for bacterial sterility, mycoplasmal sterility, and the endotoxin level (<3 EU/mL).
The recombinant adenoviral vector encoding the gene for enhanced green fluorescent protein (Ad-GFP) and mouse IFN-β (Ad-IFN-β) was constructed and produced using the AdEasy vector system, following the manufacturer's instructions (Quantum Biotechnologies, Carlsbad, CA, USA)3). MSCs were infected with a multiplicity of infection of 50 for Ad-GFP or Ad-IFN-β. Enzyme-linked immunosorbent assays (ELISA) was done to confirm the transgene expression of MSC-GFP and MSC-IFN-β. The IFN-β proteins were checked in both supernatants (Fig. 1) and the supernatants at day 3 after infection were acquired for in vitro experiments.
The GBM cell line GL26 was obtained from American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, NY, USA). All media was supplemented with 2 mmol/L L-glutamate, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum purchased from Invitrogen. Cells were incubated at 37℃ in a humidified atmosphere containing 5% CO2/95% air.
To analyze the antitumor effects of TMZ and IFN-β in the GL26 glioma tumor cell line, cells were washed twice with the medium and incubated with medium containing MSC-GFP (control), medium containing MSC-IFN-β, or medium containing MSC-IFN-β with 20 µM TMZ. After exposure to the various conditions, cells were detached by trypsinization and the viable cell population was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)1).
The established human glioma tumor cell line, GL26, was used in this experiment. C57BL/6 mice (6-8 weeks old; Harlan-Sprague-Dawley) were used in accordance with institutional guidelines under the approved protocols. For the intracranial implantation of syngeneic GL26 tumor cells in the brains of C57BL/6 mice, animals were stereotactically inoculated with 1×105 GL26 cells [in 3 µL of phosphate-buffered saline (PBS)]into the right frontal lobe (2 mm lateral and 1 mm anterior to bregma, at a depth of 2.5 mm from the skull base) using a guide-screw system (Hamilton syringe, Hamilton Company, Reno, NV, USA). To increase the uniformity of the xenograft take and growth, cells were injected simultaneously using a multiport Microinfusion Syringe Pump (Harvard Apparatus, Holliston, MA, USA). Animals were anesthetized with xylazine/ketamine during the procedure.
For the survival experiment, intracranial glioma-bearing mice were randomly divided into four groups (n=5 in each groups) after tumor implantation and treated with intratumoral injections of saline (PBS), TMZ alone, MSCs infected with Ad-IFN-β (MSC-IFN-β), or a combination of TMZ with MSC-IFN-β. TMZ was injected intraperitoneally and MSC-IFN-β was injected intracranially, directly to the tumor. The therapeutic treatments were administered on the 14th day after tumor inoculation. The brains from the treated mice (n=5/groups) were serially sectioned (20 µm-thich sections, obtained every 200 µm into the tumor) and then stained with hematoxylin and eosin. Tumor size was determined as described previously4). The section with the maximum tumor area was calculated via a computer using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA).
Apoptotic cells were visualized using a terminal deoxynucleotidyltransferased UTP nick end labeling (TUNEL) assay kit (Roche, Basel, Switzerland) developed using Cy3-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Endogenous peroxidase activity was blocked by 3% H2O2 for 10 min at room temperature. After washing with PBS, 50 µL of TUNEL reaction mixture was pipetted onto the sections, which were then incubated in a humidified chamber at 37℃ for 1 h. The reaction was stopped by adding wash buffer. In all sections, nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO, USA) for counterstaining. Apoptotic cells were also measured using a computer with the MetaMorphsoftware (Molecular Devices, Silicon Valley, CA, USA).
All data are expressed as the mean±the standard error of the mean. All statistical comparisons between the groups were determined using one-way analyses of variance and Student's t-tests. Survival analyses were conducted by a log-rank test based on the Kaplan-Meier method. Probability values of <0.05 were considered statistically significant.
To confirm the transgene expression of MSC-IFN-β, the IFN-β protein was checked in supernatants from MSC-IFN-β by enzyme-linked immunosorbent assays (Fig. 1). IFN-β protein production was well detected in the supernatants from IFN-β-transduced MSCs. To examine the involvement of MGMT in the GL26 cell line, the expression of the MGMT was analyzed by western blot and reverse transcription (RT)-polymerase chain reaction (PCR). As a result, MGMT was not detected in both examinations (data not shown). As GL26 cells were sensitive to temozolomide, to determine the combination effect with MSC-IFN-β clearly, a temozolomide concentration of 20 µM was chosen as a subtoxic dose for the following in vitro combination experiments (Fig. 2A).
To assess the combined antitumor effects of MSC-IFN-β and TMZ in the GL26 cell line, cells were incubated in culture medium containing 0-90 µL MSC-IFN-β supernatant and/or 20 µM TMZ for 72 h. Subsequently, the number of viable cells was analyzed via MTT assay. As shown in Fig. 2B, a significantly enhanced antitumor effect was observed for the combined treatment of MSC-IFN-β and TMZ (p<0.005, combined treatment vs. each single treatment). This result suggests that the combination of MSC-IFN-β and TMZ has combined antitumor effects in the glioma cell line.
To determine whether the MSC-IFN-β treatment alone and combined with TMZ showed antitumor effects on gliomas in vivo, glioma-bearing mice were treated with MSC-IFN-β, TMZ, or both. Tumor sizes were verified by histological staining (Fig. 3A). The average tumor area in each treatment group was decreased compared with PBS-treated mice. Additionally, as shown in Fig. 3A, the tumor sizes were mostly decreased in the combined treatment group. Histological analyses were performed for each treatment group, and the combined treatment was significantly better than MSC-IFN-β or TMZ alone (p<0.05, MSC-IFN-β vs. combination; p<0.01, TMZ vs. combination) (Fig. 3B). These results show that MSC-IFN-β and TMZ alone reduced the rate of tumor growth in glioma-bearing mice, while the combination of MSC-IFN-β and TMZ, had significantly better antitumor effects. Next, survival analyses were conducted, as shown in the survival curve of glioma-bearing mice (Fig. 3C). The survival of mice in the combined treatment group was significantly prolonged compared with the survival in each of the monotherapy groups (p<0.05).
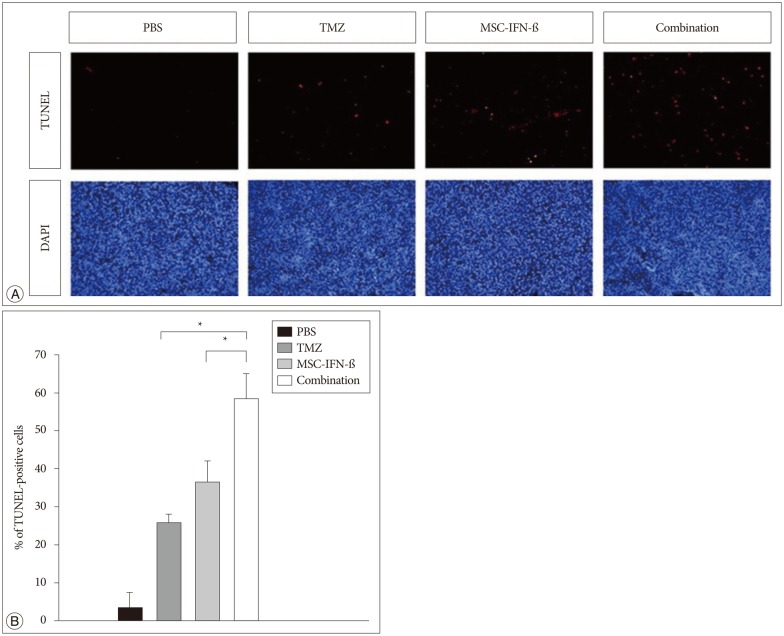
To figure out the mechanism of the combined treatment, we focused on apoptosis. To determine whether apoptotic cell death was involved in the antitumor activity of transplanted MSC-IFN-β and TMZ, TUNEL staining and counterstaining with DAPI were conducted. TUNEL staining demonstrated a significant increase in the number of apoptotic cells in the combined treatment group compared to the number in each of the monotherapy groups (p<0.05, TMZ vs. combination and MSC-IFN-β vs. combination) (Fig. 4). These results demonstrate that the antitumor activity of transplanted MSC-IFN-β and TMZ is mediated by apoptosis.
By engineering MSCs to secret IFN-β and using them in combination with TMZ, the current study demonstrates that when applied in combination, MSC-IFN-β and TMZ have significant antitumor effects in vitro, and are able to significantly reduce the tumor size and enhance the survival rates in GL26 mice. We demonstrated that the combination of MSC-IFN-β and TMZ showed significant antitumor effects in vitro, and significantly reduced the tumor size and enhanced the survival rates in vivo when compared with each of the monotherapy groups (MSC-IFN-β or TMZ alone). In addition, though it was not significant, the tumor size of glioma-bearing mice treated with MSC-IFN-β alone was reduced more than the tumor size in the TMZ monotherapy group in the in vivo experiment. This result shows the therapeutic advantage of pathotropism, which was acquired by using the MSCs. Through the intratumoral accumulation of IFN-β and the pathotropism of MSCs, combination therapy can deliver a higher concentration to the tumor and exert synergic effects.
Moreover, in the previous study that combined IFN-β and TMZ, continuous intravascular infusion of the drug was needed11). However, in this study, by intratumorally injecting MSC-IFN-β, continuous intravascular infusion was not needed to maintain the therapeutic concentration. Although our study showed advanced antitumor effects using the combination of MSC-IFN-β and TMZ for glioma treatment, we should compare these results with the outcomes of combining TMZ and IFN-β without MSCs in the future.
To evaluate the mechanism of the combination effect, we conducted TUNEL staining to demonstrate apoptotic cell death. The results showed a significant increase in the number of apoptotic cells in the group treated with the combination therapy. Further studies are needed to determine the exact mechanisms of apoptosis in this combination therapy. Other studies have suggested additional potential mechanisms, with one being the inhibition of angiogenesis2). More specifically, MSCs have been shown to suppress human glioma growth through the inhibition of angiogenesis2). Additional studies (such as signal network research) are needed to establish the mechanisms of the antitumor effect as apoptosis, as was observed in this study.
In summary, we focused on the pathotropism of MSCs and attempted use these cells as a delivery vehicle. In addition, we used IFN-β to down-regulate the expression of MGMT to enhance the antitumor effects of TMZ. Using both MSC-IFN-β and TMZ in combination, we found significantly enhanced antitumor effects in vitro and in vivo. These results suggest that the combination of MSC-IFN-β and TMZ could be considered as a new option for the treatment of malignant gliomas.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (grant number : 2014R1A2A2A01004525). It was also supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C3417).
References
1. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay : assessment of chemosensitivity testing. Cancer Res. 1987; 47:936–942. PMID: 3802100.
2. Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013; 31:146–155. PMID: 23034897.

3. Jin HT, Youn JI, Kim HJ, Lee JB, Ha SJ, Koh JS, et al. Enhancement of interleukin-12 gene-based tumor immunotherapy by the reduced secretion of p40 subunit and the combination with farnesyltransferase inhibitor. Hum Gene Ther. 2005; 16:328–338. PMID: 15812228.

4. Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008; 68:9614–9623. PMID: 19047138.

5. Lawrence YR, Mishra MV, Werner-Wasik M, Andrews DW, Showalter TN, Glass J, et al. Improving prognosis of glioblastoma in the 21st century : who has benefited most? Cancer. 2012; 118:4228–4234. PMID: 22180310.

6. Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005; 65:3307–3318. PMID: 15833864.

7. Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005; 65:7573–7579. PMID: 16140920.

8. Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, et al. A combination of IFN-beta and temozolomide in human glioma xenograft models : implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol. 2008; 61:653–659. PMID: 17564708.

9. Ryu CH, Yoon WS, Park KY, Kim SM, Lim JY, Woo JS, et al. Valproic acid downregulates the expression of MGMT and sensitizes temozolomide-resistant glioma cells. J Biomed Biotechnol. 2012; 2012:987495. PMID: 22701311.

10. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.

11. Wakabayashi T, Kayama T, Nishikawa R, Takahashi H, Yoshimine T, Hashimoto N, et al. A multicenter phase I trial of interferon-beta and temozolomide combination therapy for high-grade gliomas (INTEGRA Study). Jpn J Clin Oncol. 2008; 38:715–718. PMID: 18845525.

Fig. 1
In vitro transgene expression for MSC-IFN-β. IFN-β protein production was detected in the supernatants from IFN-β-transduced MSCs by ELISA. MSC-GFP was used as a control (data not shown). IFN-β : interferon-bata, MSC : mesenchymal stem cell, MSC-IFN-β : MSCs to secret mouse IFN-β, ELISA : enzyme-linked immunosorbent assay, GFP : green fluorescent protein.
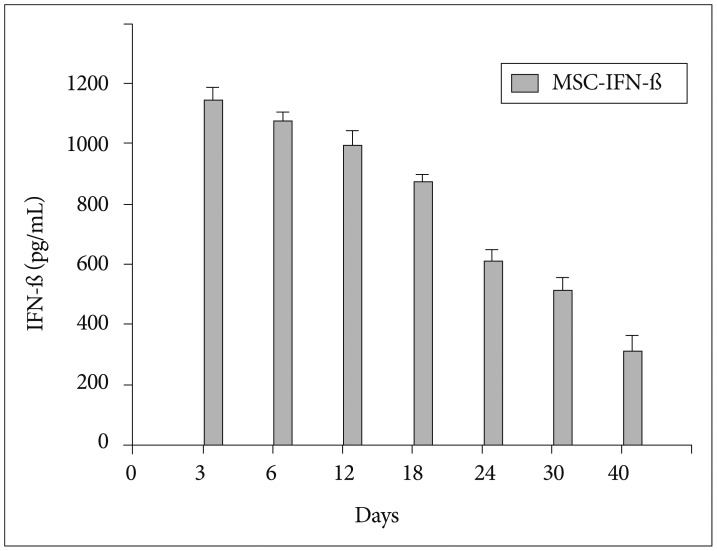
Fig. 2
Sensitivity of the GL26 glioma cell line to TMZ and IFN-β. A : The viability of glioma cells was analyzed via MTT assay 24 h, 48 h, and 72 h after TMZ (0-500 µM) treatment. B : The number of viable cells was counted 72 h after the GL26 cell line was treated with MSC-IFN-β alone and in combination with TMZ (20 µM, which was selected as subtoxic dose). The results are representative of two independent experiments. All data are represented as the mean±SEM. *p<0.05, Student's t-test. Points, mean; bars, SEM. TMZ : temozolomide, IFN-β : interferon-bata, MSC-IFN-β : MSCs to secret mouse IFN-β, SEM : standard error of the mean, MTT : 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
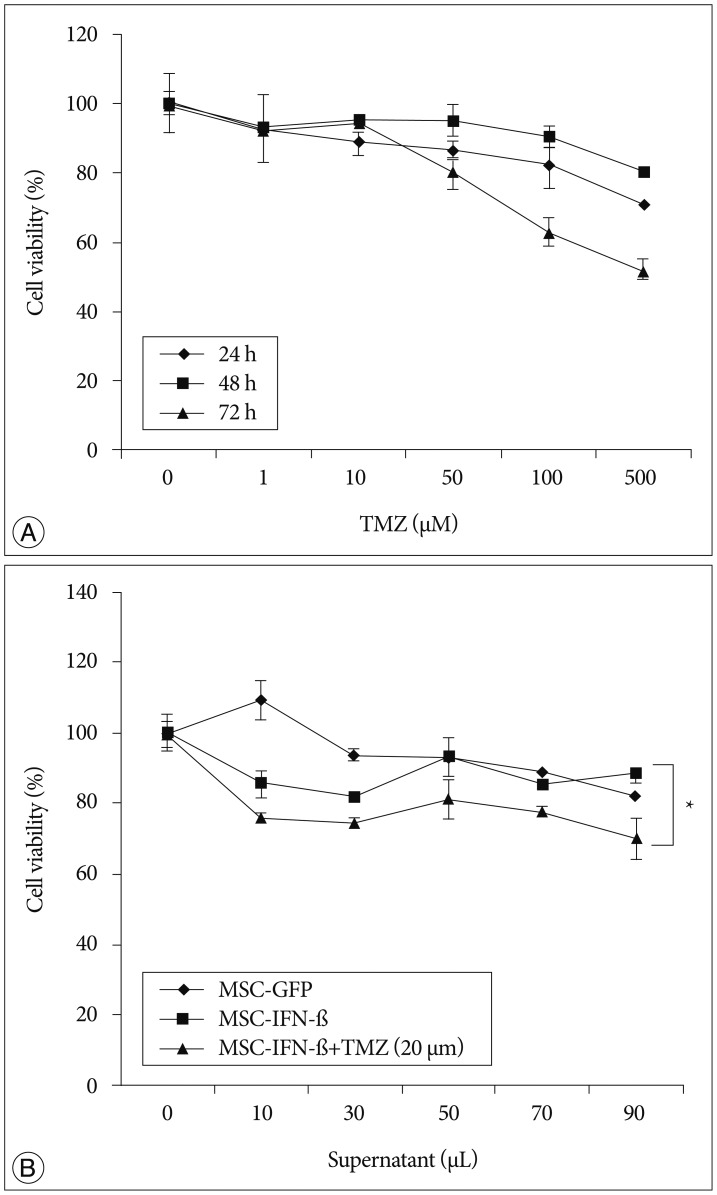
Fig. 3
In vivo effects of TMZ and MSC-IFN-β on tumor growth and the survival of intracranial glioma-bearing mice. TMZ, MSC-IFN-β, or both were injected intratumorally to glioma-bearing mice at day 14 after GL26 (1×105 cells) inoculation. The PBS was used as a control. A : Representative photographs of H&E staining from each group show tumor growth. Magnification, ×1. B : The tumor sizes in each group were determined by histological analysis at day 35 after tumor inoculation. Columns, mean; bars, SEM. *p<0.05, **p<0.01; Student's t-tests. C : Survival curve of glioma-bearing mice. At day 14 after GL26 inoculation, TMZ, MSC-IFN-β, or both were injected intraperitoneally and intratumorally. Analyses of their survival were conducted by a log-rank test based on the Kaplan-Meier method. The survival of mice in the combined treatment group was significantly prolonged compared with the survival in each of the monotherapy groups (p<0.05). The results are representative of two independent experiments (n=5 in each groups). TMZ : temozolomide, MSC-IFN-β : MSCs to secret mouse IFN-β, SEM : standard error of the mean, PBS : phosphate-buffered saline, H&E : hematoxylin and eosin.
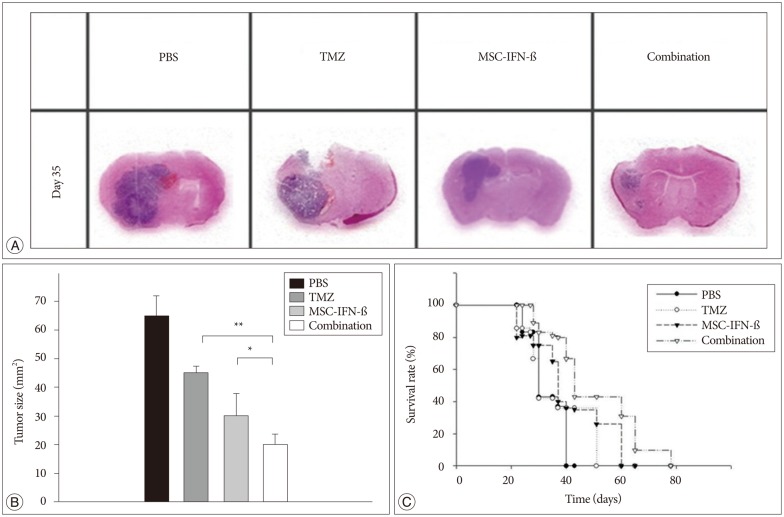
Fig. 4
Apoptotic effects of TMZ, MSC-IFN-β, or both in combination. The PBS group was used as a control. A : Apoptotic cells were detected by TUNEL staining in cryosections. TUNEL-positive nuclei (red) were stained and counterstaining was conducted with DAPI (blue). Magnification, ×200. B : The TUNEL staining intensity was quantified by computerized image analysis. The results are representative of two independent experiments (n=5 in each groups). Columns, mean; bars, SEM. *p<0.05; Student's t-test. TMZ : temozolomide, MSC-IFN-β : MSCs to secret mouse IFN-β, PBS : phosphate-buffered saline, SEM : standard error of the mean, TUNEL : terminal deoxynucleotidyltransferased UTP nick end labeling, DAPI : 4,6-diamidino-2-phenylindole.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download