Abstract
Deep brain stimulation (DBS) of the pedunculopontine nucleus (PPN) is a novel therapy developed to treat Parkinson's disease. We report a patient who underwent bilateral DBS of the PPN and subthalamic nucleus (STN). He suffered from freezing of gait (FOG), bradykinesia, rigidity and mild tremors. The patient underwent bilateral DBS of the PPN and STN. We compared the benefits of PPN-DBS and STN-DBS using motor and gait subscores. The PPN-DBS provided modest improvements in the gait disorder and freezing episodes, while the STN-DBS failed to improve the dominant problems. This special case suggests that PPN-DBS may have a unique role in ameliorating the locomotor symptoms and has the potential to provide improvement in FOG.
Deep brain stimulation (DBS) has been a widely accepted treatment modality for advanced Parkinson's disease (PD) for more than a decade. DBS could significantly improve the cardinal symptoms of PD, including tremor, rigidity and akinesia. However, in the PD patients with axial symptoms, the selection of DBS targets is still challenging. To date, the pedunculopontine nucleus (PPN) has been introduced as a potential DBS target due to the failure of globus pallidus internus (GPi)-DBS and subthalamic nucleus (STN)-DBS to improve postural instability or gait dysfunction. A basic research experiment revealed that lesions of the PPN could produce akinesia in rats and cats and that PPN stimulation increased locomotor activity6). Moreover, the low frequency stimulation of the PPN increased motor activity in a monkey model of PD11). Clinically, PPN-DBS showed the amelioration of medically intractable akinesia and gait abnormalities13,19). More recently, both open-label and blinded studies involving a series of PD patients treated with PPN-DBS have shown promising results4,14,22). We report a case whose primary symptoms were freezing of gait (FOG) and postural instability. The patient underwent DBS of the PPN and STN in our institute.
A 61-year-old male with complaints of falling, gait instability and difficulty walking was admitted to the neurology ward. He started having mild difficulty walking and using small steps to walk 2 years ago, and his symptoms have gradually deteriorated. Three months before admission, sudden freezing during gait and turns led him to fall frequently. He also experienced rigidity and a mild tremor. The neurological examination revealed bradykinesia, rigidity, postural instability, and a mild resting tremor. His expression and speech were lowered. He also had mild hypophonia. He hesitated when initiating walking. A few steps later, he would suddenly freeze and fall forward. He could not make a turn without assistance. His muscular tone was enhanced, and the Babinski sign was absent. A cranial MRI revealed mild atrophy, which was slightly more prominent in the cerebral peduncle. The administration of both levodopa and dopamine agonists improved his appendicular motor symptoms but did not change the FOG and postural instability. His levodopa dose equivalent was 750 mg/day. The duration of the levodopa benefit only lasted for 1 hour. Therefore, the patient was referred to our institution. Based on his symptoms and previous medications, the standard STN or GPi DBS would not be sufficient to improve his chief complaints.
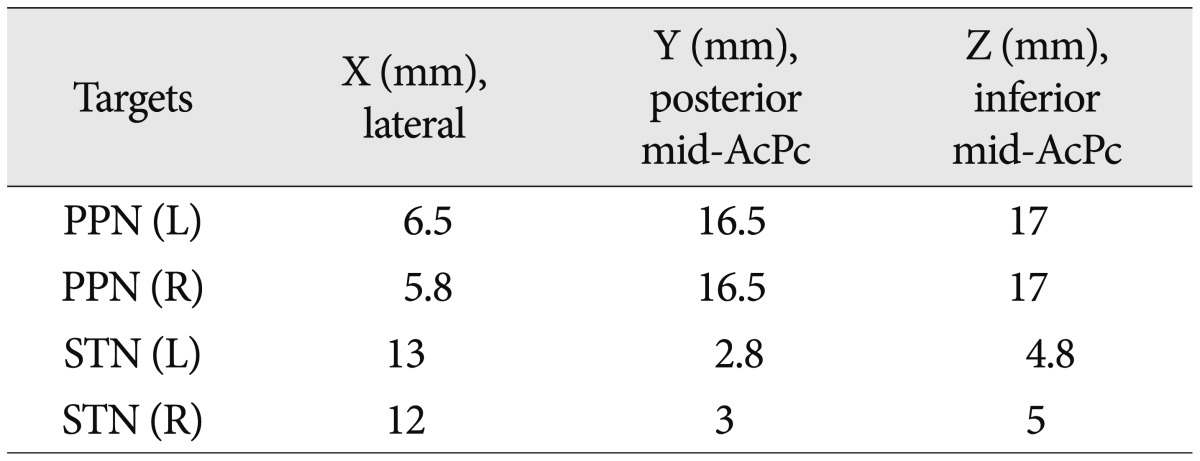
We performed a 2-stage surgery. In the first stage, 4 DBS leads (3389, Medtronic, Minneapolis, MN, USA) were implanted into the bilateral STN and PPN using MRI-based stereotactic targeting. The coordinates of the targets were refined by microelectrode recording (MER) and clinical tests. The stereotactic frame (Leksell G, Elekta Inc., USA) was fixed to the patient's head, and preoperative MR images were obtained target the STN and PPN. Zrinzo et al.26) previously published the position of the PPN relative to the anterior commissure-posterior commissure line, the midcomissural point and the ventricular floor line. For the STN, we used direct imaging targeting. The MER proceeded from 10 mm above the target and extended 5 mm below the target (Leadpoint, Medtronic) to refine both of the targets. The definitive coordinates of the PPN and STN are listed in Table 1. The electrode locations of the STN and PPN were verified by a postoperative brain MRI (Fig. 1). Then, we conducted a test stimulation trial for one week to determine which target had a better clinical effect. The IPG was implanted in stage 2.
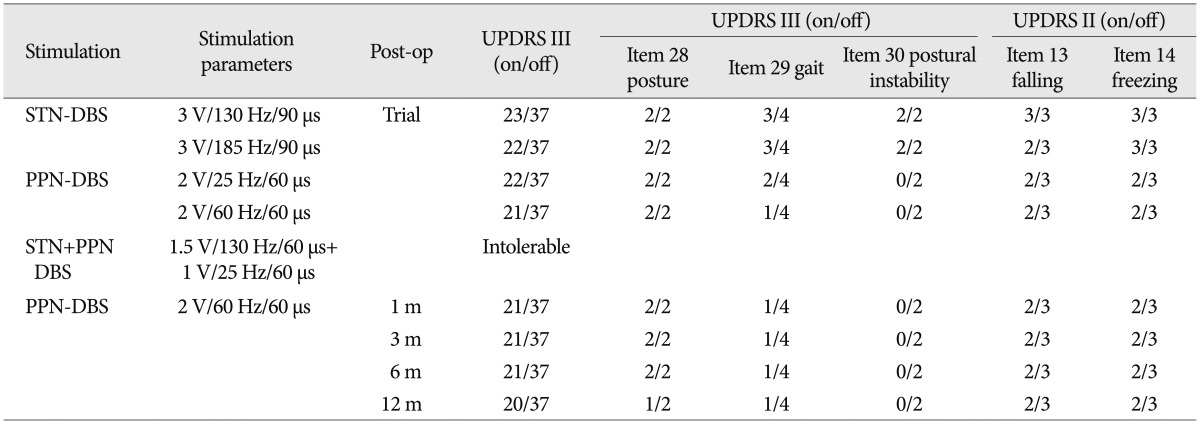
In the week of trial stimulations, bilateral PPN or STN stimulation was used to compare their effects, using daily alternation. The parameters were adjusted daily. For the PPN, the parameters ranged from 1 to 4 V in voltage, 25/60 Hz in frequency, and 60 µs in pulse width; for the STN, from 1 to 4 V in voltage, 130 to 180 Hz in frequency, and 90 µs in pulse width. After a successful trial, we selected the PPN as the final DBS target. Then, the permanent pulse generator was implanted. According to previous reports and experimental results, we used low-frequency stimulation. Nevertheless, we found that 60 Hz was more effective than 25 Hz. The final parameters were as follows : 2 V, 60 Hz, and 60 µs. During the programming, the patient did not complain of diplopia, ataxia, or pyramidal signs with the different settings. However, a high frequency or voltage led to dizziness in the patient. We also tried combination of STN and PPN stimulation, but the patient complained intolerable dizziness even in low parameters (Table 2).
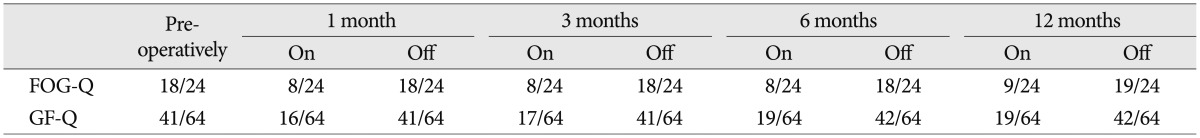
To measure the severity of the patient's symptoms, we used a variety of different scales (Table 2), including the Unified Parkinson's Disease Rating Scale (UPDRS) III, UPDRS II items 13 (falling) and 14 (freezing), UPDRS III items 28 (posture), 29 (gait), and 30 (postural instability), the freezing of gait questionnaire (FOG-Q) and the gait and falls questionnaire (GF-Q)9,10). During the trial, the STN-DBS resulted in a moderate improvement in the UPDRS III score (on/off stimulation, 22/37) but failed to improve the FOG and postural instability. In contrast, the PPN-DBS was better in ameliorating the problems with gait, postural instability, falling and FOG, although the posture score did not change. During the follow-up, the effectiveness of the PPN-DBS was stable. UPDRS 3 showed moderate improvement (on/off stimulation, 21/37) at 1, 3 and 6 months after surgery. Moreover, his posture was also improved at 12 months (on/off stimulation, 1/2) after surgery. The patient exhibited significant improvement in gait stability, reduced episodes of FOG and reduced falls. These observations were mirrored in the improved FOG-Q and GF-Q scores when the PPN stimulation was on. However, both of the scores waned at 12 months (Table 3).
Although we had doubted the diagnosis because of he started the FOG (sudden short-lasting episodes of breaks in motion and inhibition when executing a complex movement or switching from one movement to another8)), falling and bradykinesia, he had a relative good response to levodopa, which could relieve his rigidity and mild tremor. Drug-resistant FOG and postural instability are the most crippling symptoms in approximately 10% of patients diagnosed with PD7). These axial symptoms are usually disabling in patients with advanced PD16). In our patient, these symptoms emerged in the early stage. Because our patient did not appear to be suitable for the standard DBS procedure, we put the PPN-DBS into practice based on its potential benefits that were reported previously. However, the patient's symptoms also included rigidity and mild tremor, which might respond well to levodopa, and thus we decided to try a standard DBS target to ensure the efficacy. The standard targets of DBS for PD include the STN and GPi. The effectiveness of both targets has been confirmed in numerous studies. We prefer to use STN-DBS in our clinic. Although the UPDRS III scores are not significantly different between STN-DBS and GPi-DBS, STN-DBS is more advantageous. The post-operative decrease in the medication dosage was more prominent, and the stimulation voltage was lower, thereby increasing the life of IPG and reducing the cost of the DBS5). Moreover, STN-DBS showed a trend toward better motor improvement in the early stage of post-surgery compared with GPi-DBS15).
The results of the trial stimulation showed that the changes in the UPDRS III scores after the STN-DBS and PPN-DBS were similar (40.5% vs. 43.2%, respectively) but that the PPN-DBS was better in ameliorating the problems with gait, postural instability, falling and FOG. The axial symptoms were the patient's most prominent symptoms. With the parameters of 2 V, 60 Hz, and 60 µs, he did not report any side effects. STN-DBS may provide some benefits with respect to walking and postural instability25) given that our patient's gait and falling scores improved (on/off stimulation 3/4 and 2/3, respectively). However, in a large cohort of PD patients, the STN-DBS-mediated impact on the limb signs appeared to be more obvious than on the gait20). A recent meta-analysis showed that both STN-DBS and GPi-DBS improved postural instability or gait dysfunction early on but that by 2 years post-operatively, the posture and gait problems were worse than they were before the operation in patients with STN-DBS. Five years after DBS, the posture and gait problems worsened, regardless of whether STN-DBS or GPi-DBS was adopted21). Another 5-year follow-up of STN-DBS12) documented that there was some degree of deterioration in the STN-DBS-mediated impact on the axial signs and akinesia.
The PPN is located in the tegmentum of brain stem. It might play a role in the initiation and maintenance of locomotion and in motor modulation3). It receives inputs from the substantia nigra pars reticulate (SNr), STN, and GPi18) and projects into the STN, substantia nigra pars compacta, GPi, cerebellum, spinal cord and supplementary motor area (SMA, an important region for bipedal motion)2,18). In addition, the PPN has strong electrical coupling with the SMA. Analyses of PPN local field potentials and EEGs in patients have demonstrated that PPN activity changes during movement preparation and execution24). Neuron loss in the PPN has been correlated with the severity of PD symptoms27).
Although both experimental8,17) and clinical4,13,14,19,23) studies suggest that PPN-DBS is beneficial for PD patients, there are many questions left to be addressed in the future. First, all of the previous studies reported that effective stimulation is obtained at low frequencies (10-80 Hz); here, we used 60 Hz in our patient. Aravamuthan et al.1) noted the PPN is dominated by inhibitory oscillatory input from the SNr in the parkinsonian brain. Low frequency stimulation excites the PPN and thus may disrupt this pathological process and attenuate the effects of the excessive inhibitory input to the PPN in PD. However, the optimal stimulation parameters still need to be clarified. Second, most of the clinical studies have utilized bilateral PPN-DBS, but unilateral stimulation was also shown to be beneficial to PD patients14). Which of these stimulation types is better remains unknown. Third, a small open-label study by Stefani et al.22) revealed that the combination of STN and PPN stimulation proved more effective than the stimulation of either target alone and that the combination might further improve the control of the axial signs. This evidence supports the selection of previous STN-implanted patients for additional PPN stimulation. However, we did not applied the combined stimulation because of the intolerable dizziness in the patient. To date, the available data suggest that PPN-DBS may be considered relatively safe and may improve motor function. So, does PPN represent a novel safe alternative target area? Although preliminary reports are encouraging, longer term observations are required.
References
1. Aravamuthan BR, Bergstrom DA, French RA, Taylor JJ, Parr-Brownlie LC, Walters JR. Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of Parkinson's disease. Exp Neurol. 2008; 213:268–280. PMID: 18601924.

2. Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H. Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage. 2007; 37:694–705. PMID: 17644361.

3. Classen J, Schnitzler A. What does the pedunculopontine nucleus do? Neurology. 2010; 75:944–945. PMID: 20702789.

4. Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinsons disease. Brain. 2010; 133(Pt 1):205–214. PMID: 19773356.

5. Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010; 362:2077–2091. PMID: 20519680.

6. Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. 1987; 18:731–738. PMID: 3304544.

7. Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in PD : prospective assessment in the DATATOP cohort. Neurology. 2001; 56:1712–1721. PMID: 11425939.

8. Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, et al. Motor blocks in Parkinson's disease. Neurology. 1992; 42:333–339. PMID: 1736161.

9. Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000; 6:165–170. PMID: 10817956.

10. Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. 2009; 24:655–661. PMID: 19127595.

11. Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport. 2004; 15:2621–2624. PMID: 15570164.

12. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003; 349:1925–1934. PMID: 14614167.

13. Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, et al. Implantation of human pedunculopontine nucleus : a safe and clinically relevant target in Parkinson's disease. Neuroreport. 2005; 16:1877–1881. PMID: 16272871.

14. Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinsons disease. Brain. 2010; 133(Pt 1):215–224. PMID: 19846583.

15. Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord. 2010; 25:578–586. PMID: 20213817.

16. Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. CARPA Study Group. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008; 70:2241–2247. PMID: 18519873.

17. Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002; 125(Pt 11):2418–2430. PMID: 12390969.

18. Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinsons disease. Brain. 2000; 123:1767–1783. PMID: 10960043.

19. Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005; 16:1883–1887. PMID: 16272872.

20. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease : a multicentre study with 4 years follow-up. Brain. 2005; 128(Pt 10):2240–2249. PMID: 15975946.

21. St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010; 75:1292–1299. PMID: 20921515.

22. Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007; 130:1596–1607. PMID: 17251240.

23. Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery. 2011; 69:1248–1253. discussion 1254. PMID: 21725254.

24. Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, Lozano AM. Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology. 2010; 75:950–959. PMID: 20702790.

25. Voges J. Deep brain stimulation for treatment of movement disorders. J Korean Neurosurg Soc. 2003; 34:281–298.
26. Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, et al. Stereotactic localization of the human pedunculopontine nucleus : atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008; 131(Pt 6):1588–1598. PMID: 18467343.

27. Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL. The pedunculopontine nucleus in Parkinson's disease. Ann Neurol. 1989; 26:41–46. PMID: 2549845.

Fig. 1
DBS electrodes were implanted bilaterally into the STN and PPN. A and B : X-ray imaging. C and D : Transverse sections. E and F : Coronal sections. White arrows indicate PPN (C and E), black arrows indicate STN (D and F). DBS : deep brain stimulation, STN : subthalamic nucleus, PPN : pedunculopontine nucleus.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download