INTRODUCTION
Meningoencephaloceles are defined as herniation of brain tissue through a defect of the skull and can be classified according to their location
21). Common areas are occipital, cranial vault, fronto-ethmoidal and posterior fossa encephaloceles, while basal encephaloceles account for only 1.5% of cases
6). The subgroup of basal encephaloceles can further be differentiated in transethmoidal, spheno-ethmoidal, fronto-sphenoidal or sphenoorbital and transsphenoidal (temporo-sphenoidal) encephaloceles
21).
A frequent first symptom of a temporo-sphenoidal encephalocele is cerebrospinal fluid (CSF)-rhinorrhea
2,
9,
13) with consecutive recurrent meningitis
11). The majority of cases occur during childhood
19), however, a number of reports described encephaloceles that manifested in adults
2,
11,
17). Papanikolaou et al.
17) presented an overview of different origins of skull base defects, which can lead to a herniation of brain tissue (
Table 1). In this report we present the case of a 35-year-old female patient with CSF-rhinorrhea and a first-time seizure. The symptoms were caused by a temporo-sphenoidal encephalocele. Brain tissue had herniated through a bone defect of the middle cranial fossa into the lateral sphenoid sinus.
Table 1
Classification of skull base defects

Go to :

CASE REPORT
A 35-year-old female patient was referred to the Department of Otolaryngology (ENT) because of recurrent clear nasal discharge after an infection. Three months before, she had for the first time experienced an epileptic seizure. There was no history of trauma or nasal surgery.
The clinical and neurological examination showed no additional abnormalities. A β2-transferrin-test of the nasal fluid was positive, proving CSF-rhinorrhea. All blood analyses gave normal results.
Diagnostic imaging (high-resolution computed tomography, HRCT) of the paranasal sinus showed a defect of the skull base in the area of the roof of a far lateral recessus of the right sphenoid sinus. The right sphenoid sinus was filled with fluid (
Fig. 1).
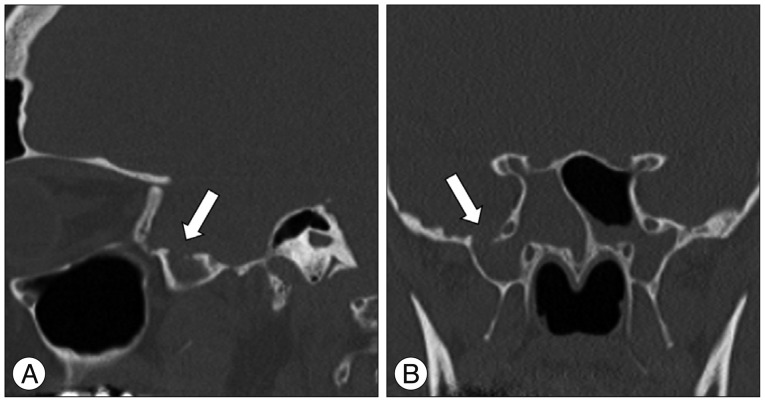 | Fig. 1Sagittal (A) and coronal (B) computed tomography scan of the skull base showing the defect (6.2×6.3×7.2 mm) in the roof of a far lateral recessus of the right sphenoid sinus leading to the middle fossa (arrows).
|
In a first therapeutic approach by the ENT department an exploration of the right sphenoid sinus via an endonasal approach was performed. CSF secretion from the posterior areas of the nasal cavity was detectable. Further exploration localized the origin of CSF in a far lateral recessus of the right sphenoid sinus, but it was not possible to directly visualize the pathological area. An ethmoidectomy was performed and it was attempted to stop the leakage by covering the bone and sealing the lateral recess with a fibrinogen und thrombin patch. In spite of this effort, the leakage of CSF persisted. An intravenous antibiotic treatment with clindamycin was initiated.
A post operative MRI revealed, besides a discontinuation of the skull base in the lateral area of the roof of the right sphenoid sinus (T1 weighted images), a herniation of brain tissue. Due to an adjacent severe inflammatory reaction and the extrinsic fibrinogen and thrombin patch, visualization of the encephalocele was challenging. The contrast-enhancing inflammatory tissue extended to the fronto-mesial temporal lobe. The fluid-sensitive sequences (T2-weighted/fluid attenuated inversion recovery) showed increased signal of the right anterior temporal lobe, which was in line with the diagnosis of encephalitis (
Fig. 2).
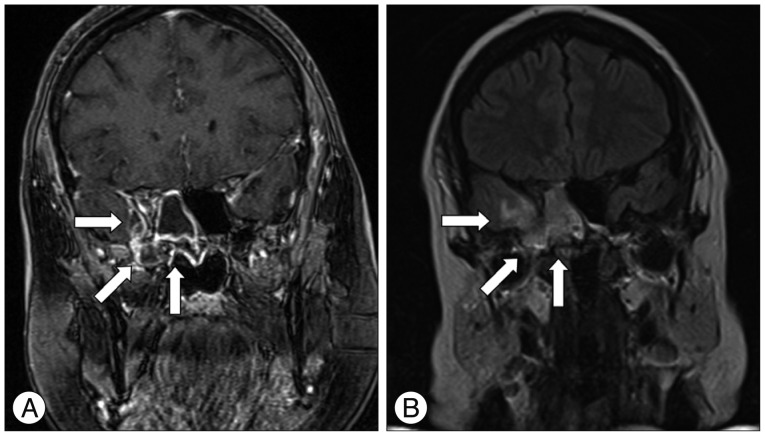 | Fig. 2A : Coronal T1-weighted magnetic resonance imaging scan of the head showing a disruption of the skull base in the area of the roof of the right sphenoid sinus where the encephalocele penetrated. A contrast enhancement in terms of an inflammatory area reaching to the fronto-mesial temporal lobe is detectable (arrows). B : Coronal T2-weighted/FLAIR magnetic resonance imaging scan of the head showing a high signal intensity of the right temporal lobe reconcilable with an encephalitis (arrows). FLAIR : fluid attenuated inversion recovery.
|
The patient was transferred to the department of neurosurgery for further treatment. On admission, neurological examination continued to show no deficit.
Because of the initial clinical course, the MRI results (inflammatory reactions of the temporal lobe) and a following operation with resulting additional irritation of the temporal lobe we decided to establish an anticonvulsive therapy with levetiracetam perioperatively.
A frontotemporal craniotomy and exploration of the middle fossa was performed with neuronavigation support. The encephalocele was visualized and resected and the dural defect was closed using galea, a fibrinogen and thrombin patch ("tachosil") and fibrin glue (
Fig. 3).
 | Fig. 3A : The middle cranial fossa is reached via a fronto-temporal approach. This way, the damaged dura and skull base become visible (arrows). B : Upon resection of the encephalocele, the dura defect is sealed with galea, tachosil, and fibrin glue (arrow).
|
Histological analysis was performed of two samples taken from the herniated brain tissue and confirmed a meningoencephalocele. Microbiological cultures were negative for microorganisms.
After craniotomy, CSF-rhinorrhea had successfully been stopped and surveillance on intensive care unit was without any complication. A post-operative control cranial computed tomography scan showed no complications. One week later, the asymptomatic patient was discharged.
Three months later, CSF-rhinorrhea reappeared without the presence of another encephalocele, as evidenced by an additional MRI. In the second attempt to close the dural defect the patient had gotten a lumbar drainage in the perioperative period. Via the old fronto-temporal approach the illustration of the middle cranial fossa revealed that the galea-fibrinogen-thrombin-patch completely disappeared. The reason for this remains uncertain, perhaps it was related to the inflammatory milieu of the adjacent tissue. Closure of the defect was done with temporalis fascia, another fibrinogen-thrombin patch and fibrin glue. Postoperative surveillance was without any complication. Four months later another seizure appeared. In addition to this, cephalgia and a papilloedema triggered further diagnostics. Invasive intracranial pressure (ICP) measurement detected a raised ICP. With dose increase of levetiracetam and a shunting procedure, we were able to solve those problems. There were no further episodes of CSF-rhinorrhea and no more seizures reported at follow-up. The application of levetiracetam had been stopped without any further appearance of seizures. In the two year follow-up MRI the inflammatory area of the fronto-mesial temporal lobe and the encephalocele were no longer detectable (
Fig. 4).
 | Fig. 4A : Coronal T1-weighted magnetic resonance imaging scan of the head showing no contrast enhancement in terms of an inflammatory area of the fronto-mesial temporal lobe (arrows). B : Coronal T2-weighted/FLAIR magnetic resonance imaging scan of the head. No abnormal signal intensity are detected in the area of the right temporal lobe (arrows). FLAIR : fluid attenuated inversion recovery.
|
Go to :

DISCUSSION
This case demonstrates a temporo-sphenoidal encephalocele that became clinically apparent by CSF-rhinorrhea and a first time seizure. The sphenoid sinus is a rather rare location for encephaloceles and depending on the topography of the encephalocele different clinical symptoms may occur. Based on the literature a classification of basal encephaloceles with regard to topography and clinical symptoms can be summarized (
Table 2). For a temporo-sphenoidal encephalocele in adulthood, an epileptic seizure is not a common clinical sign, nevertheless an association between seizures and this special entity of encephaloceles has been well documented
18,
23). A variety of structural changes of the herniated parenchyma or the adjacent cerebral tissue such as meningoangiomatosis
23), reactive gliosis or even extension of the reactive gliosis to the amygdalohippocampal lesion
16) are suggested to lead to the clinical sign of a first time seizure. In addition, there is also a body of evidence that inflammatory and immune mediators initiate seizures and epileptogenesis
7). We assume that in our case encephalitis (
Fig. 2) was responsible for the onset of epilepsy.
Table 2
Clinical symptoms of basal encephaloceles

Etiological explanations of encephaloceles are quite heterogeneous. In general, classification differs between spontaneous and acquired skull base defects
17). It is not clear whether spontaneous encephaloceles occur because of congenital defects alone. In addition, bone remodelling and reabsorption has been discussed as a reason
17). Furthermore, a differentiation of encephaloceles between congenital (childhood) and idiopathic (adulthood) encephaloceles was made
17).
The temporo-sphenoidal encephalocele often protrudes through the foramen caecum (patent craniopharyngeal canal). Catala
5) suggested that the persistence of the craniopharyngeal canal may play a crucial role in the appearance of temporo-sphenoidal encephaloceles. Besides, regarding the embryology of the sphenoid bone, it is known that in mammals the alisphenoid and the basi-pre-sphenoid are of neural crest cell origin, otherwise the orbitosphenoid and the basi-post-sphenoid derive from the cephalic mesoderm
5). In consequence, the sphenoid bone is a complex structure which contains parts of different origin regarding its derivates and genetic control
5), which makes it vulnerable to defects which may result in predilection sites for possible herniations
9). Some authors found that Sternberg's canal (membranous space in the lateral wall of the sphenoid sinus) is associated with spontaneous CSF-rhinorrhea and meningoencephaloceles in the lateral recess of sphenoid sinus
22). Interestingly, there is an aerated lateral recess of the sphenoid sinus (pterygoid recess) in up to 10% of the normal population, which has been assumed to act as a pathway between the middle fossa and the paranasal sinuses and may subsequently lead to encephalocele with potential CSF-flow through the sinus
9).
Furthermore, arachnoid pits and decreased stability of the skull base lateral to the sites of fusion of ossification centers could be responsible for encephaloceles and could result in a secondary acquired leak
3). Regarding the mechanism of the protruding brain tissue in cases of far lateral sphenoid encephalocele through these skull base defects, pithole enlargement in the skull base due to normal intracranial pressure variations might play a crucial role
9).
The treatment of sphenoid encephaloceles has been discussed controversially in the literature. A classification and differentiation by location between the rare lateral sphenoid and the more common perisellar encephaloceles seems to be useful
2,
12). For the perisellar encephaloceles endoscopic surgery might often be successful
22). Stopping the CSF flow with this variant is challenging but also less invasive than the transcranial approach
22). In contrast, for the cases of the lateral sphenoid encephaloceles, the frontotemporal approach seems to be the best choice
11,
13,
18). Hereby, a controlled resection of the encephalocele with a good overview of the operation area is possible
11,
13).
The endoscopic transnasal approach may still be successful for surgical ablation of the encephalocele and repair of the bony defect in some cases
12). However, in keeping with our case presented here, Landreneau et al.
13) reported of patients with lateral sphenoid encephaloceles which had undergone unsuccessful transnasal attempts and required a transcranial middle fossa approach for successful treatment.
Some authors reported of a sphenoidal encephalocele in adulthood with CSF-flow into the sinus where invasive ICP measurement after intervention with closure of the defect showed raised intracranial pressure
10). Besides this, mechanisms had been described, where CSF production increases because of the chronic rhinorrhea
4). For these cases a shunting procedure (ventriculoperitoneal, lumboperitoneal) solved those problems
4,
10). In our case a lumbar drainage in the perioperative period ensured the preservation of the success of the second operation regarding the arrest of the rhinorrhea. Because of raised ICP a shunting procedure was necessary.
In our opinion, a precise topographical classification of encephaloceles based on familiar classifications
21) with previous precise localisation of the encephalocele and the skull base defect with the help of HRCT and MRI is the key for the choice of the right intervention. For the choice of the suitable strategy some goals of surgery have to be considered : removal of herniation, sealing of the defect, preservation of functional neuronal tissue and, if necessary, normalisation of raised intracranial pressure or raised CSF-flow via a shunting procedure
11). Regarding this, it is necessary for the choice of the approach to precisely know the topographical and extension details of the defect, pneumatisation of the sphenoid sinus, CSF pressure, symptoms and recurrence of symptoms because of insufficient intervention and experience of the surgeon with the different strategies
11).
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download