Abstract
Vestibular schwannoma (VS) usually present the widening of internal auditory canal (IAC), and these bony changes are typically limited to IAC, not extend to temporal bone. Temporal bone invasion by VS is extremely rare. We report 51-year-old man who revealed temporal bone destruction beyond IAC by unilateral VS. The bony destruction extended anteriorly to the carotid canal and inferiorly to the jugular foramen. On histopathologic examination, the tumor showed typical benign schwannoma and did not show any unusual vascularity or malignant feature. Facial nerve was severely compressed and distorted by tumor, which unevenly eroded temporal bone in surgical field. Vestibular schwannoma with atypical invasion of temporal bone can be successfully treated with combined translabyrinthine and lateral suboccipiral approach without facial nerve dysfunction. Early detection and careful dissection of facial nerve with intraoperative monitoring should be considered during operation due to severe adhesion and distortion of facial nerve by tumor and eroded temporal bone.
Vestibular schwannoma (VS) usually arises within the internal auditory canal (IAC) from either the superior or inferior vestibular nerve. These tumors usually grow very slowly with the growth rate 0.66 to 2 mm per year6,7). With time, they cause erosion of IAC. However, the exact mechanism by which VS causes IAC erosion remains unknown. The location of tumor in IAC was closely related in erosion of IAC, tumor that arise laterally along the course of eighth nerve could tend to cause varying degree of erosion before they extend into cerebellopontine angel (CPA) cistern4). Almost all VS usually widens IAC only and could not invade the temporal bone beyond the confines of the bone of IAC. It was very rare that unilateral VS aggressively destructed temporal bone. In this report, we describe a patient without previously known bone disease who presented with aggressive temporal bone invasion of unilateral VS.
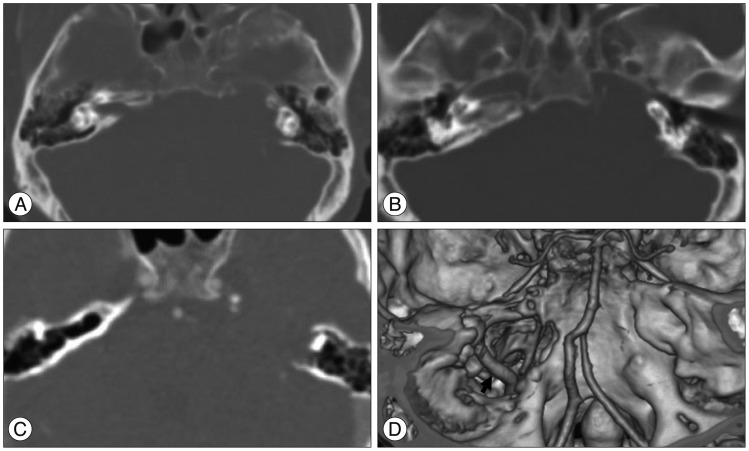
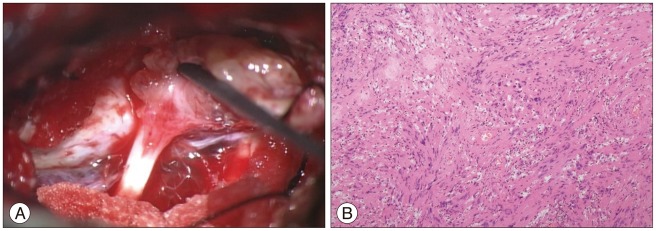
A 51-year-old male presented with progressive hearing loss of last 4 years and dizziness of few months duration. He did not have a history of a head trauma or of any brain surgery. On admission, neurological and physical examination revealed left side deafness, no facial weakness, or no other low cranial nerve deficit like loss of gag reflex, uvular deviation, tongue deviation. His facial sensory and mastication function were normal. The patient did not have any known systemic disease of bone and family history or physical characteristics of neurofibromatosis. His blood profile, including his serum calcium, phosphorus, and alkaline phosphates, was normal. CT scan showed that the tumor mass had destructed IAC completely and temporal bone to petrous apex, grown around the cochlea, reached the carotid artery in its petrous portion, extended to jugular foramen (Fig. 1). We noticed pneumatized area of opposite temporal bone corresponded to eroded area by tumor (Fig. 1C). On brain MRI, the tumor was a 4×3.8×3 cm sized, heterogeneous lobulated, well-defined mass with destruction of IAC and extended to CPA cistern. The tumor was confined to posterior fossa and not extended to Meckel's cave (Fig. 2). The combined translabyrinthine with retrosigmoid suboccipital approach was used for the resection of the tumor. At the first time of surgery, we removed the tumor piece by piece within temporal area with translabyrinthine approach, but we could not find facial nerve with this approach. So we performed additional retrosigmoid suboccipiral approach, and it allowed the exposure the lateral recess area easily and origin site of facial nerve from brain stem. After detection of facial nerve with intraoperative facial nerve monitoring, we dissected facial nerve from proximal to distal area. The dissection of the facial nerve within the temporal bone was very difficult, because the facial nerve was severely adhered to the tumor mass (Fig. 3A) and the complexity of eroded temporal bone. The tumor was extended to jugular foramen by destruction of superior wall of jugular foramen. The consistency of temporal bone was felt to be normal and the colors and the consistency of the tumor were typical of VS. We successfully achieved total removal of tumor and preserved facial nerve in continuity. Histological examination confirmed a typical schwannoma. There were no abnormal vascularity and malignant features (Fig. 3B). The postoperative course was uneventful with normal facial nerve function and the vertigo was resolved.
The patient was good condition after 18 months of follow-up without cranial nerve dysfunction. He also did not show any evidence of the recurrence or residual tumor in postoperative MRI performed at 15 months of follow up.
VS typically originates lateral to the glial-Schwann junction, and this point is usually located within the IAC, and thus can erode the IAC. But the mechanism of widening of IAC by a VS still unknown. Takada et al.5) hypothesized that the mechanism involved in the destruction of IAC is as follows : 1) the repeated strokes of the tumor caused by brain pulsation gradually eroded the bony wall of IAC (especially, that of the posterior wall). The apparently less destruction of IAC in cases with mostly cystic large tumors might be due to the restriction of this pulsatile movement. 2) Increased cerebrospinal fluid pressure on the site had some influence on the mechanism of bone erosion as also does solidity of the tumor. The bony changes are usually limited to the IAC. This case presented with aggressive pattern of invasion of temporal bone, which is more extensive than expected in case of unilateral VS. Temporal bone destruction over IAC in VS is very rare. To our knowledge, only one study has been reported in the literature. Feghali and Kantrowitz2) reported 4 cases of temporal invasion of unilateral VS, in which one cases had experienced previous schwannoma surgery. They suggested that the invasion to temporal bone occurred through two mechanism in growth of VS. One of them was that the destruction of bone in the petrous apex can actually follow the pneumatized tracts when the cortex of the IAC was eroded. The second mechanism was that a thick membranous scar had "trapped" and confined the tumor within the temporal bone and separated it from the recurrent tumor in the CPA.
We examined various factors related in the excessive erosion of temporal bone in this patient. In this case, the patient showed no signs of osteoporosis and no abnormal laboratory findings including serum level of calcium, phosphorus and alkaline phosphatase. Histologic findings demonstrated typical nature of benign schwannoma, and there were not excessive unusual vascularity nor any malignant features. He had no history of head trauma. CT scan showed pneumatized area of opposite area corresponded to destructed site by tumor. If cortex of IAC was eroded, the tumor could grow into the temporal bone through the pneumatized area. But we cannot determine why this unilateral tumor first eroded cortex of IAC and destructed temporal bone to the petrous apex before growing along the area of least resistance toward the CPA cistern.
Trigeminal schwannoma usually widen Meckel's cave and extend into middle fossa and posterior fossa, may destruct petrous apex and grow into temporal bone. MRI showed that the tumor was confined to posterior fossa and not extended to Meckel's cave. And the patient show no trigeminal nerve deficit. In facial schwannoma, tympanic segment is the most frequently involved3), so temporal bone might be destructed. Facial schwannoma is most frequently arised in originated from the tympanic segment. Facial palsy is generally regarded as the most common presentation of facial nerve schwannoma. The patient presented no facial palsy, no facial sensory change, and no facial pain. We thought that the tumor was originated form vestibular nerve.
The most common surgical approaches for VS is the translabyrinthine or suboccipital approaches1). Because of hearing loss and mass in temporal bone, we performed combined translabyrinthine with retrosigmoid suboccipital approach to detect the facial nerve earlier and remove the tumor in temporal bone. The facial nerve was severely compressed and irregularly distorted by tumor, bony edges, and crests along the invaded petrous apex. So the dissection of the facial nerve presented a particular challenge. We successfully removed the tumor without facial nerve injury.
The bony changes in VS are usually limited to widening of the IAC. Temporal bone destruction beyond the cortex of IAC in VS is very rare, which cause facial nerve compressed and distorted severely. During operation, it is very difficult to dissect and preserve the facial nerve because of uneven erosion of temporal bone. So this may cause postoperative the facial nerve palsy.
References
1. Briggs RJ, Fabinyi G, Kaye AH. Current management of acoustic neuromas : review of surgical approaches and outcomes. J Clin Neurosci. 2000; 7:521–526. PMID: 11029233.

2. Feghali JG, Kantrowitz AB. Atypical invasion of the temporal bone in vestibular schwannoma. Skull Base Surg. 1995; 5:33–36. PMID: 17171155.

3. Lipkin AF, Coker NJ, Jenkins HA, Alford BR. Intracranial and intratemporal facial neuroma. Otolaryngol Head Neck Surg. 1987; 96:71–79. PMID: 3118299.

4. Lo WWM. Tumors of the temporal bone and cerebellopontine angle. In : Som PM, Bergeron RT, editors. Head and Neck Imaging. ed 2. St. Louis: Mosby;1991. p. 420–445.
5. Takada Y, Ohno K, Hirakawa K.[Morphological and clinical study of acoustic tumor with respect to enlargement of internal auditory canal : mechanism of bone destruction]. No Shinkei Geka. 1996; 24:739–742. PMID: 8741409.
6. Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M. Growth of untreated vestibular schwannoma : a prospective study. J Neurosurg. 2012; 116:706–712. PMID: 22264178.
7. Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005; 103:59–63. PMID: 16121974.

Fig. 1
A : Internal acoustic canal is eroded to disappear totally. B : Temporal bone destruction is extended to carotid canal. C : In superior part of petrous bone, there was pneumatized area with thick cortex of right side temporal area corresponded to destructed area by tumor. D : 3 dimensional CT angiography shows IAC, superior part of jugular foramen, and carotid canal is invaded and carotid artery (arrow) is exposed. IAC : internal auditory canal.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download