Abstract
Objective
The purposes of this article are to present 5 cases of intracranial meningioma with leptomeningeal dissemination (LD) and investigate the characteristics of this disease.
Methods
We present a retrospective case series of 5 females at our institutions (age ranged 21-72 years, mean 54.6 years) diagnosed with LD of an intracranial meningioma after surgery between 1998 and 2013. A database search revealed 45 cases with LD of meningioma in the English literature. Characteristic features were analyzed and compared.
Results
The incidence rate at our institutions of LD of meningioma was 0.9% (5/534). World Health Organization (WHO) grade was distributed as follows: I : 2, II : 2, and III : 1. Time to LD ranged from 2.5 months to 6.9 years; the patient with WHO grade III had the shortest interval to LD. The patient with an intraventricular meningioma (WHO grade II) had the second shortest interval to LD (1.7 years), and simultaneously revealed both LD and extraneuronal metastases. Four of 5 patients showed a disease progression, with the survival ranging from 1 month to 3.8 years after LD. Based on the literature, the initial tumor was an intraventricular meningioma in 9 patients, and their time to LD was shorter on average (mean 1.9 years). Histologically, 26 of 45 (58%) were initially diagnosed with a WHO grade II or III meningioma, and 6 of 19 patients (32%) with WHO grade I revealed malignant transformation.
Intracranial meningiomas account for 15 to 20% of all primary brain tumors, and most of them are benign [World Health Organization (WHO) grade I]46). Aggressive (WHO grade II) or malignant (WHO grade III) meningiomas overall constitute less than 10% of meningiomas, and they are rarely associated with distant metastases37,50). If metastases are found, the sites are usually the liver, lungs, pleura, and lymph nodes1,4,15,34,43). This finding suggests that distant metastases usually occur through a blood-borne route. However, some leptomeningeal disseminations (LDs) of intracranial meningioma through cerebrospinal fluid (CSF) have been reported in the literature1,2,5,7,8,9,10,13,14,16,17,18,19,20,21,22,23,24,25,26,27,29,34,35,36,39,42,43,44,45,47,48,49). In this report, we summarized our experience with 5 intracranial meningiomas with LD through CSF and reviewed the relevant literature to evaluate the characteristics of patients with LD and discuss predisposing factors.
Between 1998 and 2013, 5 patients with intracranial meningiomas demonstrated evidence of LD through CSF at our institutions. We retrospectively reviewed the medical records, imaging studies, and histopathological findings to determine the general features of the patients, presentation of LD, possible predisposing factors, treatment, and outcomes.
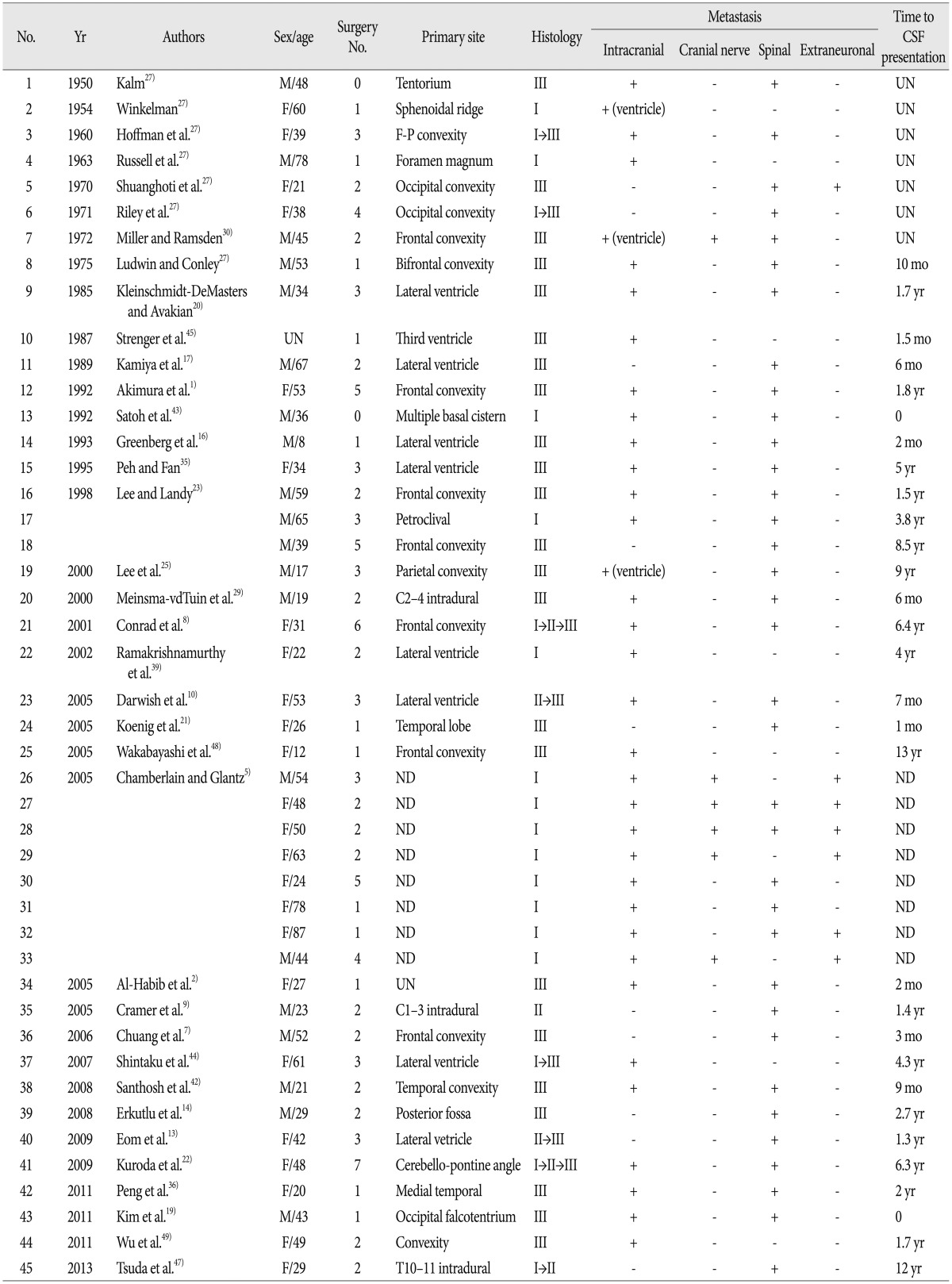
We also reviewed PubMed, Medline, Embase, and ISI Web of Knowledge using the keywords 'meningioma', 'cerebrospinal fluid', 'leptomeningeal', 'dissemination' and 'metastasis'. All articles were reviewed, and relevant articles were collected. Forty-five cases of LD through CSF were found from 36 articles since 1950. All cases were reviewed using the same protocol from our own series.
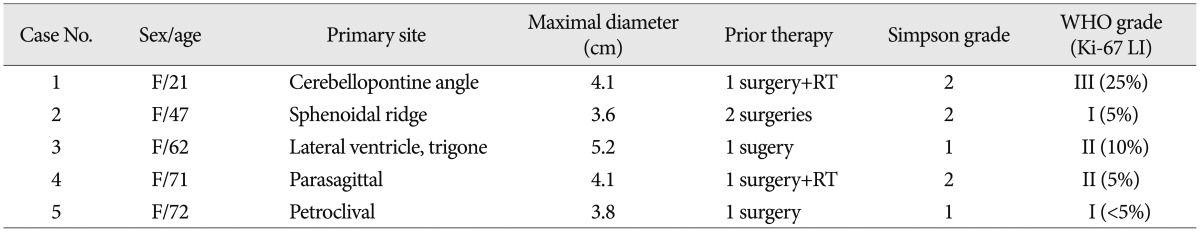
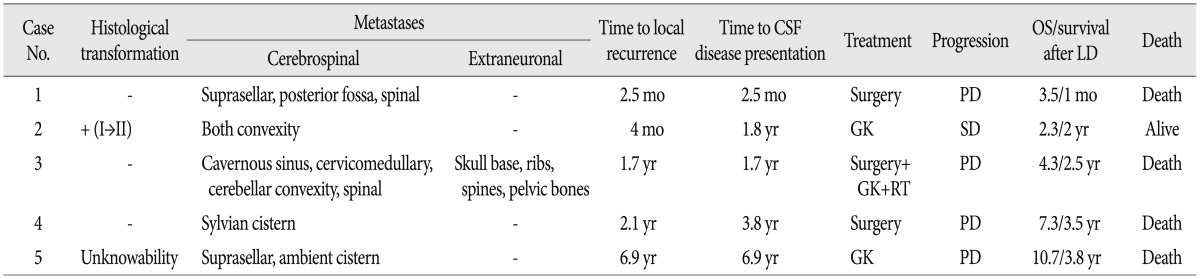
Five in 534 consecutive patients with a surgically resected intracranial meningioma at our institutions were confirmed to have a meningioma with LD. Therefore, the incidence of LD was 0.9% (5/534). Clinical data are summarized in Table 1. All patients were female, with age ranging from 21 to 72 years (mean age, 54.6 years). Initial tumors were located in the posterior fossa in two cases, and the parasagittal region, sphenoidal ridge and lateral ventricle in one, respectively. The mean maximal diameter of the tumor was 4.1 cm (range, 3.6 to 5.2 cm). All patients had undergone surgery and achieved grossly total tumor resection (Simpson grade 1 or 2) before LD.
All histological specimens after the first operation confirmed a meningioma, and subtypes were assigned following the WHO classification in accordance with histological features and the mitotic index. Pathological examinations demonstrated two cases each of WHO grade I and II, respectively, and one case of WHO grade III (Table 1). There was immunoreactivity for epithelial membrane antigen and vimentin in all patients. The values of Ki-67 labelling index (LI) ranged from less than 5% to 25%.
The mean time to local recurrence after the first surgery was 2.3 years (range, 2.5 months-6.9 years) and the mean time to LD after the first surgery was 2.9 years (range, 2.5 months-6.9 years) (Table 2). Three of five cases had local recurrence and LD occur at the same time. Interestingly, the patient with a malignant meningioma (WHO grade III) had the shortest interval to LD (2.5 months).
Intracranial LD was seen in all cases, and two cases among them additionally showed spinal LD. One patient with intracranial and spinal LD had an intraventricular meningioma and showed multiple extraneuronal metastases, including areas of the skull base, rib and pelvic bone.
Four patients underwent a second surgery for local recurrence or metastatic tumors. Three patients had to receive additional radiotherapy or Gamma-Knife radiosurgery. After a second operation, three pathologic examinations revealed the same findings as the initial specimens, and there was one case of histologically aggressive transformation from WHO grade I to II.
Overall survival ranged from 3.5 months to 10.7 years with a mean of 4.9 years, and survival after LD ranged from 1 month to 3.8 years with a mean of 2.4 years. Four patients showed disease progression and eventually died from the disease. One patient initially with WHO grade I was still alive and stable at 2 years follow-up.
Because of relatively small sample size and incomplete data from the literature, it was impossible to perform adequately statistical analysis and validate the predicting variables including gender, age, treatments, tumor location and grading. However, possible factors that may predispose meningiomas to leptomeningeal dissemination were evaluated.
No patients in this series had an association with neurofibromatosis or familial disease. All of them received surgery before the LD. Although the Ki-67 LI varied, 3 out of 5 (60%) showed aggressive and malignant histological features, and one patient in particular had a histological transformation from WHO grade I to II. In addition, one patient with a primary intraventricular meningioma concurrently manifested intracranial and spinal LD and extraneuronal metastases.
Based on the literature (Table 3), the initial location of meningiomas with LD was reported in 36 cases and was distributed as follows : convexity (14 cases, 39%), intraventricular (9 cases, 25%), posterior fossa (6 cases, 17%), and other (7 cases, 19%). With regard to time to LD, intraventricular meningiomas (mean 23.6 months, range from 1.5 months to 5 years) showed a shorter time interval compared to other intracranial (mean 39.2 months, range from 0 to 13 years) and spinal meningiomas (mean 55.6 months, range from 6 months to 12 years).
Histologically, 26 out of 45 cases (58%) were either WHO grade II (3 cases) or III (23 cases). Additionally, 8 cases were confirmed as histologic transformations (6 cases, from WHO grade I to II or III; 2 cases, from WHO grade II to III).
A 23-year-old female presented with mild headache and nausea for 2 weeks. Neurological examination was unremarkable. Brain magnetic resonance imaging (MRI) revealed a 4.1-cm-diameter meningioma on right cerebellopontine angle (Fig. 1A). She underwent craniotomy and removal of tumor (Simpson grade II resection) (Fig. 1B). Histopathlogical findings demonstrated a malignant meningioma (WHO grade III). Adjuvant radiotherapy (54 Gy in 1.8 Gy daily fractions) was performed. Approximately 2.5 months later, follow-up MRI showed the local recurrence (Fig. 1C) and new lesions in the posterior fossa, suprasellar and spinal region (Fig. 1D). Spinal MRI revealed multiple leptomeningeal disseminations (Fig. 1E). She underwent a second surgery for a suprasellar tumor and additional radiotherapy was scheduled, but she was dead one month after surgery.
A 47-year-old female was transferred to our hospital for further evaluation of a 3.6-cm-sized an extraaxial mass on a left sphenoidal ridge that was discovered on computed tomography (CT). She complained of a headache, but neurological exam was unremarkable. MRI showed a left sphenoidal ridge meningioma (Fig. 2A). Surgery was performed (Simpson grade II resection), and a histopathological finding demonstrated WHO grade I. Four months later, brain MRI showed the local recurrence (Fig. 2B) and a second surgery was performed. The second pathological examination confirmed the histological transformation (from WHO grade I to II). Approximately 18 months later, follow-up MRI revealed multiple meningiomas around bilateral convexity (Fig. 2C, D). Gamma-Knife radiosurgery was performed for multiple masses. Now 2 months after radiosurgery, clinical course of the patient was uneventful.
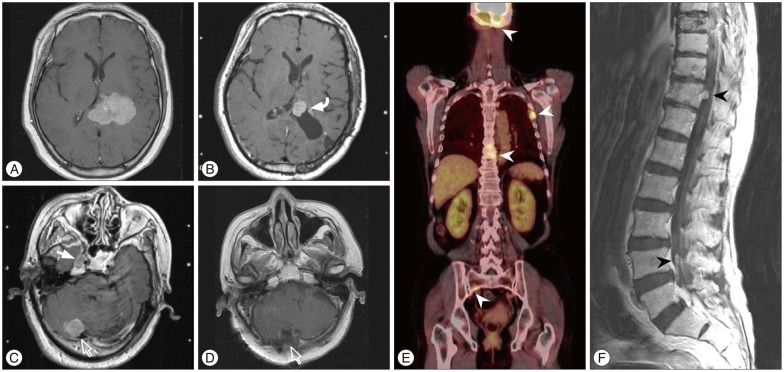
A 62-year-old female presented with dizziness and tingling sense on right limbs for several months. Neurological exam revealed paresthesia on right face and limbs. MRI showed a 5.2-cm-diameter meningioma in the trigone of the left lateral ventricle (Fig. 3A). She underwent surgery (Simpson grade I resection) and a histopathological finding revealed an atypical meningioma. Approximately 1.7 years later, brain MRI showed the local recurrence (Fig. 3B) and new lesions in the right cavernous sinus and posterior fossa (Fig. 3C). Gamma-Knife radiosurgery was administered to the local recurrence and cavernous sinus lesion. And, a second surgery for posterior fossa tumors and focal radiotherapy were performed additionally (Fig. 3D). Four months later, positron emission tomography-CT showed multiple extraneuronal metastases in the skull base, ribs, spines and pelvic bone (Fig. 3E). Spinal MRI revealed multiple leptomeningeal disseminations (Fig. 3F). Approximately 30 months after leptomeningeal dissemination, she was dead.
Distant metastases of meningiomas are rare, and most have been reported to occur from malignant (WHO grade 3) tumors12). The pathways of metastases are various. The most common destinations for metastasis are the liver, lungs, pleura, and lymph nodes, which indicates that a hematogenous route through the jugular vein may be the most frequent pathway for metastasis11). Less common are metastases through the paravertebral venous plexus, lymphatic vessels and cerebrospinal fluid, which are reported in a small minority of cases. Particularly, metastases through CSF are extremely rare1). However, considering that meningiomas arise from the arachnoid villi, tela choroidea or leptomeninges, and are naturally exposed to CSF during their growth or at the time of surgical intervention, it is difficult to explain the rarity of LD through CSF.
Since 1950, only 45 cases of postoperative LD of meningioma through CSF have been reported in the literature (Table 3). Despite the extreme rarity of LD of meningioma, CSF is a certain route for meningioma dissemination. Russell and Rubinstein41) reported that CSF should be expected to provide a good culture medium for tumor cells, and it is apparent that tumor friability may play a great role in determining the potential for CSF dissemination. Chamberlain and Glantz5) also reported a large study regarding LD through CSF in 2005. Two hundred meningiomas were followed in their prospective study (median time, 8.5 years). Eight out of 200 patients (4%) had LD and positive CSF cytology.
In light of the physiologic pathways involving CSF, the risk of intraventricular meningiomas to metastasize to leptomeninges should be high. However, only 9 cases with intraventricular meningiomas were described in the literature. Miller and Ramsden30) conjectured the dynamics of the CSF system might prevent fragment formation and the settlement of tumor cells. However, considering the number of intraventricular meningiomas with LD and the rarity of meningioma metastases, 9 cases (25% of 36 cases where initial location was reported) represent a relatively significant sample size. And, as mentioned above, time to LD involving intraventricular meningiomas is the shortest when compared to other intracranial and spinal meningiomas. These findings suggest that the ventricular location is an important site for the LD through CSF of meningiomas.
Histologically, several factors are predictive for the metastasis of meningiomas, according to the literature. They include characteristic features of the malignant tumor, such as high cellularity, cellular heterogeneity, high mitosis rate, nuclear pleomorphism, tumor necrosis, and invasion of adjacent blood vessels12,15). Although these factors do not explain metastasis of benign meningiomas, the proportion of malignant meningiomas with LD is high when compared to the general proportion of subtypes of meningioma. Twenty-six (58%) out of 45 cases were WHO grade II or III. With regard to histologic transformation, 6 of 8 histologic transformations were aggressive transformations (from WHO grade I to II or III) (Table 3). In the presented cases, 3 cases were grade II or III, and one case with WHO grade I had histological transformation to WHO grade II at the time of LD. These findings suggest that a malignant meningioma tends to metastasize to the leptomeninges more frequently.
The genetic characterization of meningiomas involving histologic transformation has been studied during the past decade. The cumulative acquisition of genetic alterations in meningiomas might be related to the development of LD, especially in cases with malignant transformation. The most common allelic loss in benign meningiomas is alteration in the long arm of chromosome 22 (22q), and this alteration is considered to be the earliest initiating event in meningioma formation3). Whereas allelic loss at other loci, such as 1p, 3q, 6q, 10q, 14q, 17q, and 18q, is associated with tumor progression from the benign state to the atypical and anaplastic meningiomas31). Recently, Nakane et al.33) suggested that meningiomas similarly progress from the benign to the malignant state by the accumulation of genetic alterations such as 1p loss of heterozygosity and p73/RASSF1A promotor methylation. However, the genetic alterations of meningiomas with LD have not yet been reported in the literature owing to the rarity of LD. The study of a larger number of meningiomas with LD could enable the elucidation of the genetic aspect.
Meningiomas are naturally exposed to CSF at the time of surgical intervention. Theoretically, surgical resection might initiate metastatic spread. However, this theory seems improbable since the incidence is so low in the face of so many tumors being operated on, and there have been cases describing LD through CSF for which there was no surgical intervention39). On the other hand, the surgical resection theoretically could influence malignant transformation of meningiomas. Koenig et al.21) reported that growth factors at the site of surgical trauma exacerbate a genetic or environmental predilection for malignancy. This theory was tested in an experimental rat model in which neonatal rats were exposed to intravenously administered high-dose nitrogen-based carcinogens and underwent surgical trauma to the brain. Compared to a control group, which was also exposed to carcinogens, twice as many neoplasms, including gliomas and vestibular schwannomas, developed at the site of injury in the surgical trauma group32).
When meningiomas are observed at multiple sites, it is difficult to distinguish whether the focus is a multiple tumor or a metastatic tumor. Multiple meningiomas are defined as two or more tumors appearing simultaneously or sequentially in the same patient, and the incidence of multiple meningiomas ranges from 1% to 16% of all meningiomas40). Multiple meningiomas usually occur in association with neurofibromatosis type 2, but also have been described in families with no evidence of neurofibromatosis28). However, almost all examples in the literature mentioned that meningioma with LD is not associated with neurofibromatosis and familial multiple meningiomas. And, in our cases, LD is certainly secondary to postoperative seeding through CSF, because histopathlogical features of additional biopsies were similar to the first biopsy and there was no familial history. Therefore, neurofibromatosis and familial multiple meningioma may have little role in LD.
Spinal LDs usually occur in malignant primary brain tumors, and those that arise from intracranial meningiomas are extremely rare. Based on the literature, meningiomas accompanied by spinal LDs were reported in 35 of 45 cases (78%). Especially, 6 out of 9 cases (67%) with intraventrciular meningomas were accompanied by spinal LDs. In addition, 4 of those cases were WHO grade III and the other 2 cases were confirmed as malignant transformation from WHO grade II to III. In the intraventricular meningiomas with LD, the median and mean time interval between local recurrence and spinal LD was 6 months and 10.5 months, respectively (range from 0 to 21 months). And, the mean time interval between intracranial LD and spinal LD was 4 months. In the present cases, a patient with an atypical intraventricular meningioma showed local recurrence and intracranial LD at the same time, and spinal LD was developed approximately 4 months after intracranial LD (Case 3). Considering the literature and the present case, screening spinal MRI could be carefully considered between 6 months and 12 months after local recurrence of a non-benign intraventricular meningioma. When intracranial LDs of a malignant intraventricular meningioma are diagnosed, whole spinal MRI should be performed immediately, and follow-up spinal MRI could be carefully considered between 3 months and 6 months after the time of LD.
So far, multimodal treatments, including multiple surgeries, chemotherapy (systemic or regional intraventricular chemotherapy) and radiotherapy (whole brain, spine and involved-field radiotherapy to sites) were performed for patients with LD. However, the prognoses in the reported literature were dismal. This is because there have been few therapeutic options after a tumor grows following administration of radiation-based therapy. In addition, a few patients received chemotherapy, and the received chemotherapy regimens were limited. Recently, as a new effort, Chamberlain et al.5,6) aggressively treated with concurrent systemic chemotherapy (temozolomide, irinotecan, interferon-α, cyclophosphamide, and bevacizumab) and regional chemotherapy (intraventricular liposomal cytosine arabinoside, busulfan, and thiotepa). Although there was a short median survival5,6), 3 patients with disease stability were alive at the time of last follow-up and one patient's survival was 39 months. This finding revealed a possibility for more effective chemotherapy for a patient with LD. Also, Pradat et al.38) demonstrated that some patients with meningeal gliomatosis may benefit from a concurrent radiotherapy to symptomatic areas and chemotherapy with agents that cross the blood-brain barrier or are delivered directly into the CSF. In the present cases, the above treatments were not administered because the patients' condition had deteriorated rapidly. However, in the future, an effective combination of radiotherapy and chemotherapy should be considered for meningiomas with LD through CSF.
Intracranial meningioma with LD is extremely rare. And, the pathogenesis for LD of meningioma remains unknown. Although our study does not demonstrate statistically significant conclusions, we generated two hypotheses based on the presented cases and a literature review. Firstly, intraventricular location and histologically aggressive features of meningiomas may be the predisposing factors for LD. Secondly, although the reported prognoses of LD of meningioma were poor, aggressively adjuvant chemotherapy or effective combination of chemo- and radiotherapy should be considered.
Acknowledgements
We thank Wade Martin of Medical Research International for his critical review of this manuscript.
This research was supported by Kyungpook National University Research Fund 2010.
References
1. Akimura T, Orita T, Hayashida O, Nishizaki T, Fudaba H. Malignant meningioma metastasizing through the cerebrospinal pathway. Acta Neurol Scand. 1992; 85:368–371. PMID: 1621502.

2. Al-Habib A, Lach B, Al Khani A. Intracerebral rhabdoid and papillary meningioma with leptomeningeal spread and rapid clinical progression. Clin Neuropathol. 2005; 24:1–7. PMID: 15696777.
3. Baser ME, Poussaint TY. Age associated increase in the prevalence of chromosome 22q loss of heterozygosity in histological subsets of benign meningioma. J Med Genet. 2006; 43:285–287. PMID: 15980114.

4. Bigner SH, Johnston WW. The cytopathology of cerebrospinal fluid. II. Metastatic cancer, meningeal carcinomatosis and primary central nervous system neoplasms. Acta Cytol. 1981; 25:461–479. PMID: 7025541.
5. Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005; 103:1427–1430. PMID: 15690330.

6. Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004; 62:1210–1212. PMID: 15079029.

7. Chuang HC, Lee HC, Cho DY. Intracranial malignant meningioma with multiple spinal metastases--a case report and literature review : case report. Spine (Phila Pa 1976). 2006; 31:E1006–E1010. PMID: 17172988.
8. Conrad MD, Schonauer C, Pelissou-Guyotat I, Morel C, Madarassy G, Deruty R. Recurrent lumbosacral metastases from intracranial meningioma. Report of a case and review of the literature. Acta Neurochir (Wien). 2001; 143:935–937. PMID: 11685626.

9. Cramer P, Thomale UW, Okuducu AF, Lemke AJ, Stockhammer F, Woiciechowsky C. An atypical spinal meningioma with CSF metastasis : fatal progression despite aggressive treatment. Case report. J Neurosurg Spine. 2005; 3:153–158. PMID: 16370305.

10. Darwish B, Munro I, Boet R, Renaut P, Abdelaal AS, MacFarlane MR. Intraventricular meningioma with drop metastases and subgaleal metastatic nodule. J Clin Neurosci. 2004; 11:787–791. PMID: 15337153.

11. Dogan S, Sahin S, Taskapilioglu O, Aksoy K, Adim S. Multiple metastatic malignant meningioma : a case report. Zentralbl Neurochir. 2004; 65:141–145. PMID: 15306979.

12. Enam SA, Abdulrauf S, Mehta B, Malik GM, Mahmood A. Metastasis in meningioma. Acta Neurochir (Wien). 1996; 138:1172–1177. discussion 1177-1178. PMID: 8955436.

13. Eom KS, Kim DW, Kim TY. Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression : a case report. Clin Neurol Neurosurg. 2009; 111:619–623. PMID: 19482417.

14. Erkutlu I, Buyukhatipoglu H, Alptekin M, Berkyurek E, Tutar E, Gok A. Spinal drop metastases from a papillary meningioma : a case report and review of the literature : utility of CSF sampling. Med Oncol. 2009; 26:242–246. PMID: 18937081.

15. Figueroa BE, Quint DJ, McKeever PE, Chandler WF. Extracranial metastatic meningioma. Br J Radiol. 1999; 72:513–516. PMID: 10505022.

16. Greenberg SB, Schneck MJ, Faerber EN, Kanev PM. Malignant meningioma in a child : CT and MR findings. AJR Am J Roentgenol. 1993; 160:1111–1112. PMID: 8470588.
17. Kamiya K, Inagawa T, Nagasako R. Malignant intraventricular meningioma with spinal metastasis through the cerebrospinal fluid. Surg Neurol. 1989; 32:213–218. PMID: 2772810.

18. Kepes JJ, MacGee EE, Vergara G, Sil R. A case report. Malignant meningioma with extensive pulmonary metastases. J Kans Med Soc. 1971; 72:312–316. PMID: 5571871.
19. Kim JP, Park BJ, Lim YJ. Papillary meningioma with leptomeningeal seeding. J Korean Neurosurg Soc. 2011; 49:124–127. PMID: 21519503.

20. Kleinschmidt-DeMasters BK, Avakian JJ. Wallenberg syndrome caused by CSF metastasis from malignant intraventricular meningioma. Clin Neuropathol. 1985; 4:214–219. PMID: 4064387.
21. Koenig MA, Geocadin RG, Kulesza P, Olivi A, Brem H. Rhabdoid meningioma occurring in an unrelated resection cavity with leptomeningeal carcinomatosis. Case report. J Neurosurg. 2005; 102:371–375. PMID: 15739568.

22. Kuroda H, Kashimura H, Ogasawara K, Sugawara A, Sasoh M, Arai H, et al. Malignant intracranial meningioma with spinal metastasis--case report. Neurol Med Chir (Tokyo). 2009; 49:258–261. PMID: 19556736.
23. Lee TT, Landy HJ. Spinal metastases of malignant intracranial meningioma. Surg Neurol. 1998; 50:437–441. PMID: 9842867.

24. Lee W, Chang KH, Choe G, Chi JG, Chung CK, Kim IH, et al. MR imaging features of clear-cell meningioma with diffuse leptomeningeal seeding. AJNR Am J Neuroradiol. 2000; 21:130–132. PMID: 10669237.
25. Lee WH, Chen A, Chao DG, Harn HJ, Lin SZ. Malignant meningioma with rhabdoid transformation. Zhonghua Yi Xue Za Zhi (Taipei). 2000; 63:492–497. PMID: 10925541.
26. Lucey BP, Tihan T, Pomper MG, Olivi A, Laterra J. Spinal meningioma causing diffuse leptomeningeal enhancement. Neurology. 2003; 60:350–351. PMID: 12552066.

27. Ludwin SK, Conley FK. Malignant meningioma metastasizing through the cerebrospinal pathways. J Neurol Neurosurg Psychiatry. 1975; 38:136–142. PMID: 1151393.

28. Maxwell M, Shih SD, Galanopoulos T, Hedley-Whyte ET, Cosgrove GR. Familial meningioma : analysis of expression of neurofibromatosis 2 protein Merlin. Report of two cases. J Neurosurg. 1998; 88:562–569. PMID: 9488313.

29. Meinsma-vdTuin M, Molenaar WM, Mooij JJ. Spinal papillary meningioma : a case report and review of the literature. Acta Neurochir (Wien). 2000; 142:703–708. PMID: 10949447.
30. Miller AA, Ramsden F. Malignant meningioma with extracranial metastases and seeding of the subarachnoid space and the ventricles. Pathol Eur. 1972; 7:167–175. PMID: 4635599.
31. Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas : a review. Neurosurgery. 2005; 57:538–550. discussion 538-550. PMID: 16145534.

32. Morantz RA, Shain W. Trauma and brain tumors : an experimental study. Neurosurgery. 1978; 3:181–186. PMID: 703937.
33. Nakane Y, Natsume A, Wakabayashi T, Oi S, Ito M, Inao S, et al. Malignant transformation-related genes in meningiomas : allelic loss on 1p36 and methylation status of p73 and RASSF1A. J Neurosurg. 2007; 107:398–404. PMID: 17695396.

34. Noterman J, Depierreux M, Raftopoulos C, Brotchi J. [Metastases of meningioma. Apropos of 2 cases]. Neurochirurgie. 1987; 33:184–189. PMID: 3614492.
35. Peh WC, Fan YW. Case report : intraventricular meningioma with cerebellopontine angle and drop metastases. Br J Radiol. 1995; 68:428–430. PMID: 7795983.

36. Peng J, Liang ZG, Li KC. Intracranial malignant meningioma with cerebrospinal fluid dissemination : a case report. Chin Med J (Engl). 2011; 124:1597–1599. PMID: 21740827.
37. Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. "Malignancy" in meningiomas : a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999; 85:2046–2056. PMID: 10223247.
38. Pradat PF, Hoang-Xuan K, Cornu P, Mokhtari K, Martin-Duverneuil N, Poisson M, et al. Treatment of meningeal gliomatosis. J Neurooncol. 1999; 44:163–168. PMID: 10619500.
39. Ramakrishnamurthy TV, Murty AV, Purohit AK, Sundaram C. Benign meningioma metastasizing through CSF pathways : a case report and review of literature. Neurol India. 2002; 50:326–329. PMID: 12391463.
40. Rosa L, Luessenhop AJ. Multiple meningiomas. In : Schmidek HH, editor. Meningiomas and Their Surgical Management. Philadelphia: WB Saunders;1991. p. 83.
41. Russell DS, Rubinstein LJ. Pathology of tumors of the nervous system. ed 4. Baltimore: Williams and Wilkins;1977. p. 89–91.
42. Santhosh K, Kesavadas C, Radhakrishnan VV, Thomas B, Kapilamoorthy TR, Gupta AK. Rhabdoid and papillary meningioma with leptomeningeal dissemination. J Neuroradiol. 2008; 35:236–239. PMID: 18325590.

43. Satoh T, Kageyama T, Yoshimoto Y, Kamata I, Date I, Motoi M. [Intrathecal dissemination of meningiomas; a case report]. No Shinkei Geka. 1992; 20:805–808. PMID: 1630573.
44. Shintaku M, Hashimoto K, Okamoto S. Intraventricular meningioma with anaplastic transformation and metastasis via the cerebrospinal fluid. Neuropathology. 2007; 27:448–452. PMID: 18018478.

45. Strenger SW, Huang YP, Sachdev VP. Malignant meningioma within the third ventricle : a case report. Neurosurgery. 1987; 20:465–468. PMID: 3574625.
46. Sutherland GR, Florell R, Louw D, Choi NW, Sima AA. Epidemiology of primary intracranial neoplasms in Manitoba, Canada. Can J Neurol Sci. 1987; 14:586–592. PMID: 3500769.
47. Tsuda K, Akutsu H, Yamamoto T, Ishikawa E, Saito A, Nakai K, et al. Benign spinal meningioma without dural attachment presenting delayed CSF dissemination and malignant transformation. Brain Tumor Pathol. 2013; 30:185–191. PMID: 22915133.

48. Wakabayashi K, Suzuki N, Mori F, Kamada M, Hatanaka M. Rhabdoid cystic papillary meningioma with diffuse subarachnoid dissemination. Acta Neuropathol. 2005; 110:196–198. PMID: 15981015.

49. Wu YT, Ho JT, Lin YJ, Lin JW. Rhabdoid papillary meningioma : a clinicopathologic case series study. Neuropathology. 2011; 31:599–605. PMID: 21382093.
50. Younis GA, Sawaya R, DeMonte F, Hess KR, Albrecht S, Bruner JM. Aggressive meningeal tumors : review of a series. J Neurosurg. 1995; 82:17–27. PMID: 7815129.
Fig. 1
A : Preoperative enhanced magnetic resonance imaging (MRI) showing a right cerebellopontine angle meningioma. B : Postoperative MRI displaying Simpson grade II resection of the tumor. C and D : Approximately 2.5 months later, follow-up MRI revealing the local recurrence (curved arrow) and new lesions in the posterior fossa (closed arrow), suprasellar (open arrow) and spinal region (arrowheads). E : Spinal MRI demonstrating multiple leptomeningeal disseminations (arrowheads).
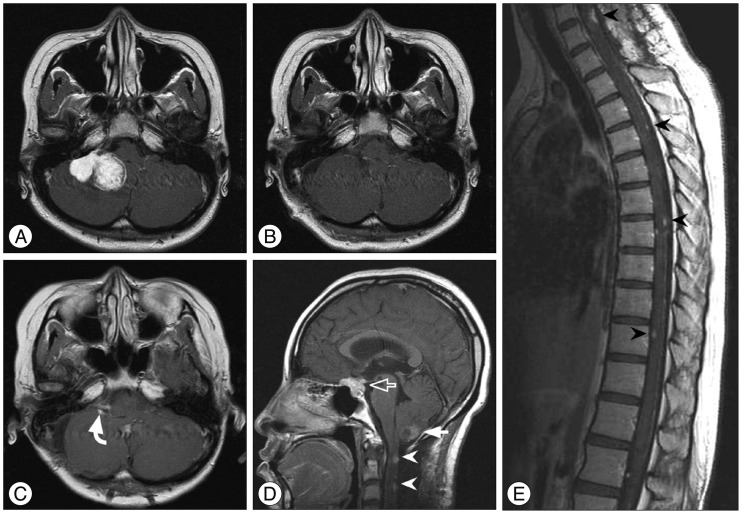
Fig. 2
A : Preoperative MRI displaying a left sphenoidal ridge meningioma. B : Four months later, MRI showing the local recurrence (arrowhead). C and D : Approximately 18 months later, MRI revealing multiple meningiomas around bilateral convexity (arrowheads).
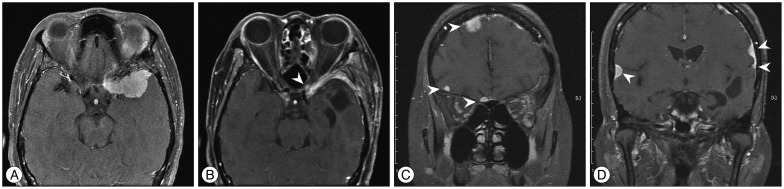
Fig. 3
A : Preoperative MRI demonstrating a meningioma in the trigone of the left lateral ventricle. B : Approximately 1.7 years later, brain MRI showing the local recurrence (curved arrow). C : New lesions in the right cavernous sinus (closed arrow) and posterior fossa (open arrow) on enhanced MRI. D : Postoperative MRI after a second surgery for posterior fossa (open arrow). E : Four months later, positron emission tomography-CT showing multiple extraneuronal metastases in the skull base, ribs, spines and pelvic bone (arrowheads). F : Spinal MRI revealing leptomeningeal dissemination (arrowheads).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download