1. Chalouhi N, Jabbour P, Gonzalez LF, Dumont AS, Rosenwasser R, Starke RM, et al. Safety and efficacy of endovascular treatment of basilar tip aneurysms by coiling with and without stent assistance : a review of 235 cases. Neurosurgery. 2012; 71:785–794. PMID:
22743359.

2. Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999; 212:348–356. PMID:
10429689.

3. Colby GP, Paul AR, Radvany MG, Gandhi D, Gailloud P, Huang J, et al. A single center comparison of coiling versus stent assisted coiling in 90 consecutive paraophthalmic region aneurysms. J Neurointerv Surg. 2012; 4:116–120. PMID:
21990478.

4. Gordhan A, Invergo D. Stent-assisted aneurysm coil embolization : safety and efficacy at a low-volume center. Neurol Res. 2011; 33:942–946. PMID:
22080995.

5. Gory B, Klisch J, Bonafé A, Mounayer C, Beaujeux R, Moret J, et al. Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms : mid-term results from the SOLARE Study. Neurosurgery. 2014; 75:215–219. discussion 219. PMID:
24818784.

6. Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Viñuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000; 93:561–568. PMID:
11014533.

7. Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997; 87:944–949. PMID:
9384409.

8. Izar B, Rai A, Raghuram K, Rotruck J, Carpenter J. Comparison of devices used for stent-assisted coiling of intracranial aneurysms. PLoS One. 2011; 6:e24875. PMID:
21966374.

9. Kim DJ, Suh SH, Lee JW, Kim BM, Lee JW, Huh SK, et al. Influences of stents on the outcome of coil embolized intracranial aneurysms : comparison between a stent-remodeled and non-remodeled treatment. Acta Neurochir(Wien). 2010; 152:423–429. PMID:
19806305.

10. Kim JW, Park YS. Endovascular treatment of wide-necked intracranial aneurysms : techniques and outcomes in 15 patients. J Korean Neurosurg Soc. 2011; 49:97–101. PMID:
21519497.

11. Kwon BJ, Seo DH, Ha YS, Lee KC. Endovascular Treatment of Wide-necked Cerebral Aneurysms with an Acute Angle Branch Incorporated into the Sac : Novel methods of Branch Access in 8 Aneurysms. Neurointervention. 2012; 7:93–101. PMID:
22970418.

12. Kwon OK, Kim SH, Kwon BJ, Kang HS, Kim JH, Oh CW. Endovascular treatment of wide-necked aneurysms by using two microcatheters : techniques and outcomes in 25 patients. AJNR Am J Neuroradiol. 2005; 26:894–900. PMID:
15814940.
13. Kwon OK, Kim SH, Oh CW, Han MH, Kang HS, Kwon BJ, et al. Embolization of wide-necked aneurysms with using three or more microcatheters. Acta Neurochir (Wien). 2006; 148:1139–1145. discussion 1145. PMID:
16990989.

14. Lee SY, Chae KS, Rho SJ, Choi HK, Park HS, Ghang CG. Clinical and Angiographic Outcomes of Wide-necked Aneurysms Treated with the Solitaire AB Stent. J Cerebrovasc Endovasc Neurosurg. 2013; 15:158–163. PMID:
24167794.

15. Li MH, Gao BL, Fang C, Gu BX, Cheng YS, Wang W, et al. Angiographic follow-up of cerebral aneurysms treated with Guglielmi detachable coils : an analysis of 162 cases with 173 aneurysms. AJNR Am J Neuroradiol. 2006; 27:1107–1112. PMID:
16687553.
16. Moret J, Cognard C, Weill A, Castaings L, Rey A. The "Remodelling Technique" in the Treatment of Wide Neck Intracranial Aneurysms. Angiographic Results and Clinical Follow-up in 56 Cases. Interv Neuroradiol. 1997; 3:21–35. PMID:
20678369.

17. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms : 11 years' experience. J Neurosurg. 2003; 98:959–966. PMID:
12744354.

18. Ng P, Khangure MS, Phatouros CC, Bynevelt M, ApSimon H, McAuliffe W. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils : analysis of midterm angiographic and clinical outcomes. Stroke. 2002; 33:210–217. PMID:
11779912.

19. Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms : clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010; 41:110–115. PMID:
19959540.
20. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID:
12775880.

21. Shapiro M, Babb J, Becske T, Nelson PK. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms : a literature review. AJNR Am J Neuroradiol. 2008; 29:1777–1781. PMID:
18719039.

22. Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK. Stent-supported aneurysm coiling : a literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012; 33:159–163. PMID:
22033717.

23. Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Balloon-assisted coil embolization of intracranial aneurysms : incidence, complications, and angiography results. J Neurosurg. 2006; 105:396–399. PMID:
16961133.

24. Tamatani S, Ito Y, Abe H, Koike T, Takeuchi S, Tanaka R. Evaluation of the stability of aneurysms after embolization using detachable coils : correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol. 2002; 23:762–767. PMID:
12006273.
25. Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002; 50:239–249. discussion 249-250. PMID:
11844258.

26. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; 19:604–647. PMID:
3363593.

27. Wanke I, Doerfler A, Schoch B, Stolke D, Forsting M. Treatment of wide-necked intracranial aneurysms with a self-expanding stent system : initial clinical experience. AJNR Am J Neuroradiol. 2003; 24:1192–1199. PMID:
12812954.
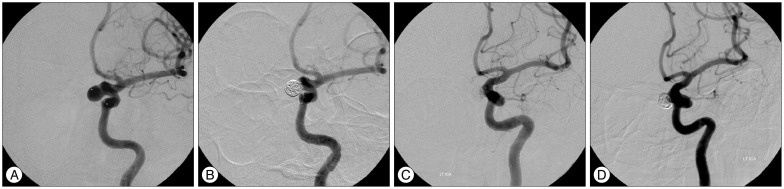
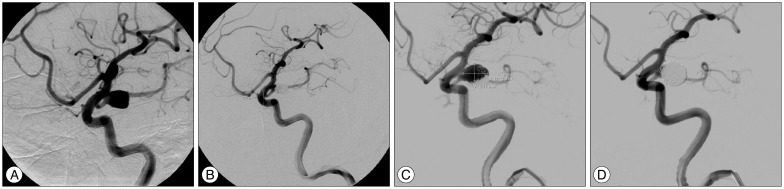
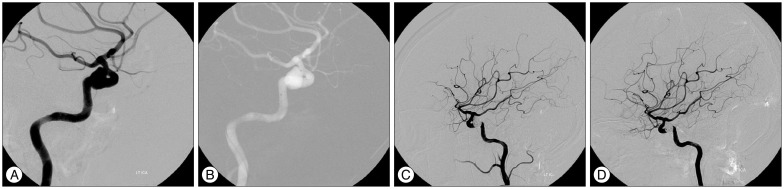
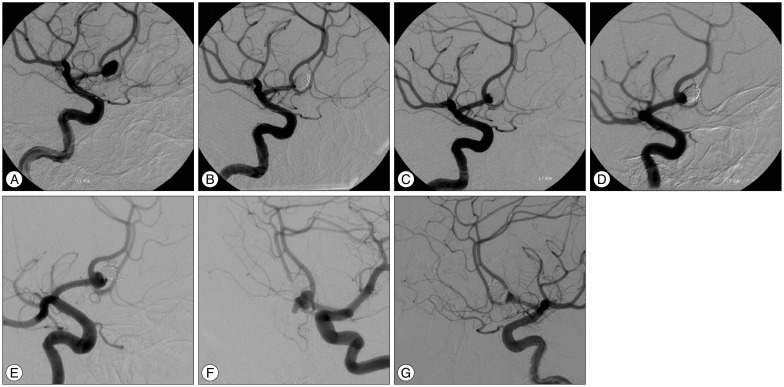




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download