Abstract
Hemifacial spasm (HFS) is a clinical syndrome characterized by unilateral facial nerve dysfunction. The usual cause involves vascular compression of the seventh cranial nerve, but compression by an artery passing through the facial nerve is very unusual. A 20-year-old man presented with left facial spasm that had persisted for 4 years. Compression of the left facial nerve root exit zone by the anterior inferior cerebellar artery (AICA) was revealed on magnetic resonance angiography. During microvascular decompression surgery, penetration of the distal portion of the facial nerve root exit zone by the AICA was observed. At the penetrating site, the artery was found to have compressed the facial nerve and to be immobilized. The penetrated seventh cranial nerve was longitudinally split about 2 mm. The compressing artery was moved away from the penetrating site and the decompression was secured by inserting Teflon at the operative site. Although the facial spasm disappeared in the immediate postoperative period, the patient continued to show moderate facial weakness. At postoperative 12 months, the facial weakness had improved to a mild degree. Prior to performing microvascular decompression of HFS, surgeons should be aware of a possibility for rare complex anatomy, such as compression by an artery passing through the facial nerve, which cannot be observed by modern imaging techniques.
Hemifacial spasm (HFS) is a clinical syndrome characterized by unilateral facial nerve dysfunction, such as intermittent painless, involuntary and spasmodic contractions of muscles. HFS typically begins as spontaneous paroxysms of eye twitching, and progresses to involve other muscles innervated by the facial nerve27). In 1970, Jannetta et al. popularized the concept of compression of the facial nerve at its exit from the brain stem by one or more arteries or veins as the most common etiology of HFS, and favored microvascular decompression as an effective treatment19,20). The most common arteries involved in HFS, in order of most frequent observation, include the anterior inferior cerebellar artery (AICA), posterior inferior cerebellar artery (PICA) and vertebral arteries. Other less frequent etiologies have been described to cause HFS including aneurysm, arteriovenous malformation, tumor, ectatic vertebrobasilar arteries and Paget's disease of the skull. However, in review of the literature, we were unable to find any report of HFS associated with an artery that had passed through the facial nerve. Therefore, we report a case of HFS associated with penetration of the facial nerve by the AICA.
A 20-year-old male patient presented with a 4-year history of progressive HFS beginning with left orbicularis oris spasms, often triggered by eating or drinking. He described the spasms as progressing from the mouth to the eye and eventually involving the platysma, which is unusual in typical HFS. The patient's hearing was normal and he exhibited no cranial nerve palsy or neurological deficits.
Concentric needle electromyography examination of the left orbicularis oculi and orbicularis oris revealed irregular bursting discharge at rest and normal motor unit potential on volition. Also, evidence of synkinesis in the orbicularis oculi and mentalis muscle was observed during supraorbital nerve stimulation. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) revealed a compression of the left facial nerve root exit zone by the AICA (Fig. 1).
Based on the above, microvascular decompression was selected for treatment. During the operation, penetration of the distal portion of the facial nerve root exit zone by the AICA was observed. At the penetrating site, the artery was found to have compressed the facial nerve and to be immobilized. After discussing the risk of facial paresis with the patient's family, the penetrated seventh cranial nerve was longitudinally split about 2 mm. The compressing artery was moved away from the penetrating site and the decompression was secured by inserting Teflon at the operative site (Fig. 2).
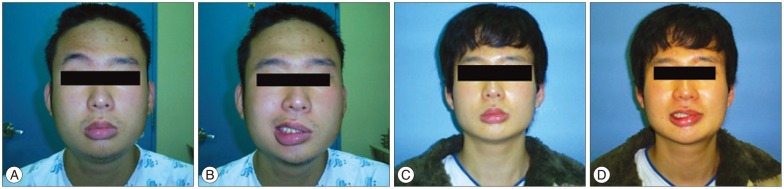
Although the facial spasm disappeared in the immediate postoperative period, the patient continued to show moderate facial weakness. At postoperative 4 months, the facial weakness had improved to a mild degree (Fig. 3).
HFS is a benign condition, characterized by unilateral paroxysmal chaotic and clonic spasms of the orbicularis oculi, paranasal, perioral and platysma muscles41,45). Most cases of HFS are due to a vascular pathology that compresses the exiting nerve root of nerve VII7,35). In a previous study, Han et al.15) reviewed 1642 cases of HFS to evaluate unusual possible causes and clinical presentations, and reported that 1633 cases (99.4%) involved neurovascular compression. The other unusual causes were a secondary causative structural lesion (0.5%), a tumor (0.4%) and a vascular malformation (0.1%)15).
Upon literature review, the most common causes of HFS, besides neurovascular compression, were cerebellopontine angle tumors (epidermoid, arachnoid cyst, lipoma, and vestibular schwannoma)2,3,9,15,26,38), and other unusual causes including cerebellopontine angle medullary venous malformations15,24), Paget's disease16,25), occipital falcine meningioma5), cerebellopontine angle meningioma11), acoustic schwannoma11), pontine glioma45), fourth ventricle ganglioglioma6), pontine infarction43), syringobulbia4), multiple sclerosis42), trauma29), hypothyroidism13), idiopathic intracranial hypertension37), vertebrobasilar ectasia21), craniovertebral anomalies28), glomus jugular tumor23), parotid gland8,11,12,32), and arterial hypertension33). A few cases of HFS alone or in combination with trigeminal neuralgia have been reported as a false localizing sign in patients who had a contralateral posterior fossa mass or acoustic neuroma30,31). This present case involved an unusual cause of HFS, and there have been no reports of HFS caused by penetration of the nerve by an artery.
From an embryological point of view, the beginning of the facial nerve, the facioacoustic primordium, appears during the third week of gestation. The facial nerve then splits into two parts at the end of the fourth week and is complete by the fifth to sixth gestational week36). The AICA appears later, in the fourth to fifth month of gestation, when all definitive communications of the facial nerve are established22,34). Accordingly, we surmised that the penetration of the facial nerve may have happened as the AICA attempted to pass through the split in the facial nerve that appears at the fifth to sixth gestational week.
The introduction of MRI and MRA has improved the detection of neurovascular conflicts (vascular contact and/or compression of the root exit zone of the facial nerve)1,14,39). High-resolution MRI and MRA techniques highly sensitive for neurovascular conflict are frequently needed14,39). These imaging studies generally show great sensitivity in detecting unusual causes of HFS; however, in the present case, such imaging studies failed to reveal the penetration of the nerve by the AICA, as shown in Fig. 1.
Patients with HFS usually present between 40 and 50 years of age10,40,44). In most cases of HFS, the initial site of onset is the orbicularis oculi muscle, although a few cases have reported the cheek and the perioral region as the initial site of onset10,18,40). Over months to years, the spasms spread gradually to other muscles innervated by the ipsilateral facial nerve in a synchronous manner. However, as we described above, this case was unusual in that the onset and clinical presentation of HFS did not follow the normal progression of symptoms, as the spasms progressed from the mouth to the eye, and eventually involved the platysma. Such unusual clinical presentations could indicate unusual causes of HFS2,11,17).
In this case, although the penetration of the distal portion of the facial nerve root exit zone by the AICA was longitudinally incised after discussing the risk of facial paresis with the patient's family, it is important to consider which is more beneficial to leave without facial spasm or leave without facial weakness. The authors considered that the importance for each person could be different. The compressing artery was moved away from the penetrating site and the decompression was secured by inserting Teflon at the operative site. Although the facial spasm disappeared in the immediate postoperative period, the patient continued to show moderate facial weakness after 4 months of follow-up. In retrospect, transposition of the vessel structure to alleviate compression of the nerve may have been an alternative option, instead of the longitudinal incision of the facial nerve we performed. However, this would not be an option through which to completely relieve the facial spasm, but rather to partially relieve with the preserve of facial weakness.
References
1. Adler CH, Zimmerman RA, Savino PJ, Bernardi B, Bosley TM, Sergott RC. Hemifacial spasm : evaluation by magnetic resonance imaging and magnetic resonance tomographic angiography. Ann Neurol. 1992; 32:502–506. PMID: 1456734.

2. Altinörs N, Kars Z, Cepoğlu C. Rare causes of hemifacial spasm. Report of two cases. Clin Neurol Neurosurg. 1991; 93:155–158. PMID: 1652399.
3. Auger RG, Piepgras DG. Hemifacial spasm associated with epidermoid tumors of the cerebellopontine angle. Neurology. 1989; 39:577–580. PMID: 2927684.

4. Barraquer-Bordas , Zamora S, Abello-Vila P. [Hemifacial spasm and bilateral central deafness in the clinical picture of possible syringobulbia]. Rev Esp Otoneurooftalmol Neurocir. 1955; 14:17–22. PMID: 14395752.
5. Bhayani R, Goel A. Occipital falcine meningioma presenting with ipsilateral hemifacial spasm : a case report. Br J Neurosurg. 1996; 10:603–605. PMID: 9115659.
6. Bills DC, Hanieh A. Hemifacial spasm in an infant due to fourth ventricular ganglioglioma. Case report. J Neurosurg. 1991; 75:134–137. PMID: 2045898.
7. Coakham HB. The microvascular treatment of trigeminal neuralgia, hemifacial spasm and glossopharyngeal neuralgia. In : Robertson JT, Coakham HB, Robertson JH, editors. Cranial base surgery. New York: Churchill Livingston;2000. p. 543–564.
8. Destee A, Bouchez B, Pellegrin P, Warot P. Hemifacial spasm associated with a mixed benign parotid tumour. J Neurol Neurosurg Psychiatry. 1985; 48:189–190. PMID: 2984333.

9. Digre K, Corbett JJ. Hemifacial spasm : differential diagnosis, mechanism, and treatment. Adv Neurol. 1988; 49:151–176. PMID: 3278539.
10. Ehni G, Woltman HW. Hemifacial spasm : review of one hundred and six cases. Arch Neurol Psychiatry. 1945; 53:205–211.
11. Gálvez-Jiménez N, Hanson MR, Desai M. Unusual causes of hemifacial spasm. Semin Neurol. 2001; 21:75–83. PMID: 11346028.

12. Gandon J, Trotoux J, Peynègre R, André J, Brasnu D. [Study of a series of 158 parotidectomies and histological problems in mixed tumors of salivary glands (author's transl)]. Ann Otolaryngol Chir Cervicofac. 1979; 96:261–280. PMID: 225977.
13. Genís D, Ricart W, Fernández-Real JM, Dávalos A, Molins A, Ferrándiz M. Hemifacial spasm and hypothyroidism. Lancet. 1993; 342:1112. PMID: 8105329.

14. Girard N, Poncet M, Caces F, Tallon Y, Chays A, Martin-Bouyer P, et al. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology. 1997; 39:46–51. PMID: 9121649.

15. Han IB, Chang JH, Chang JW, Huh R, Chung SS. Unusual causes and presentations of hemifacial spasm. Neurosurgery. 2009; 65:130–137. discussion 137. PMID: 19574834.

16. Ing EB, Savino PJ, Bosley TM, Sergott RC, Kelepouris N. Hemifacial spasm and osteitis deformans. Am J Ophthalmol. 1995; 119:376–377. PMID: 7872405.

17. Inoue T, Maeyama R, Ogawa H. Hemifacial spasm resulting from cerebellopontine angle lipoma : case report. Neurosurgery. 1995; 36:846–850. PMID: 7596519.
18. Janetta PJ. Cranial Rhizopathies : Neurological Surgery. ed 3. Philadelphia: W.B. Saunders;1990. p. 4169–4182.
19. Jannetta PJ. Microsurgical exploration and decompression of the facial nerve in hemifacial spasm. Cuff Topics Surg Res. 1970; 2:217–220.
20. Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977; 47:321–328. PMID: 894338.
21. Kerber CW, Margolis MT, Newton TH. Tortuous vertebrobasilar system : a cause of cranial nerve signs. Neuroradiology. 1972; 4:74–77. PMID: 4343431.

22. Kier EL. Development of cerebral vessels. In : Newton TH, Potts DG, editors. Radiology of the skull and brain. St. Louis: Mosby;1974. p. 1089–1141.
23. Kiley MA, Voyvodic F, Burns RJ. An unusual cause of hemifacial spasm. J Clin Neurosci. 1999; 6:349–351. PMID: 10844764.

24. Kim Y, Tanaka A, Kimura M, Yoshinaga S, Tomonaga M. Arteriovenous malformation in the cerebellopontine angle presenting as hemifacial spasm--case report. Neurol Med Chir (Tokyo). 1991; 31:109–112. PMID: 1715038.

25. Linazasoro G, Martí Massó JF. Paget's disease and hemifacial spasm. Neurology. 1992; 42:1643–1644. PMID: 1641175.

26. Loeser JD, Chen J. Hemifacial spasm : treatment by microsurgical facial nerve decompression. Neurosurgery. 1983; 13:141–146. PMID: 6888693.
27. Maroon JC. Hemifacial spasm. A vascular cause. Arch Neurol. 1978; 35:481–483. PMID: 666603.
28. Maroun FB, Jacob JC, Weir BK, Mangan MA. Hemifacial spasm and craniovertebral anomaly. Can J Neurol Sci. 1990; 17:424–426. PMID: 2276101.

29. Martinelli P, Giuliani S, Ippoliti M. Hemifacial spasm due to peripheral injury of facial nerve : a nuclear syndrome? Mov Disord. 1992; 7:181–184. PMID: 1584239.

30. Matsuura N, Kondo A. Trigeminal neuralgia and hemifacial spasm as false localizing signs in patients with a contralateral mass of the posterior cranial fossa. Report of three cases. J Neurosurg. 1996; 84:1067–1071. PMID: 8847575.

31. Nishi T, Matsukado Y, Nagahiro S, Fukushima M, Koga K. Hemifacial spasm due to contralateral acoustic neuroma : case report. Neurology. 1987; 37:339–342. PMID: 3808320.

32. Nussbaum M. Hemifacial spasm associated with benign parotid tumor. Ann Otol Rhinol Laryngol. 1977; 86(1 Pt 1):73–74. PMID: 189664.

33. Oliveira LD, Cardoso F, Vargas AP. Hemifacial spasm and arterial hypertension. Mov Disord. 1999; 14:832–835. PMID: 10495046.

34. Osborn AG. Diagnostic cerebral angiography. ed 2. Philadelphia: Lippincott Williams & Wilkins Publishers;1999.
35. Rapanà A, Guida F, Conti C, Rizzo G, Trincia G. Ependymoma of the fourth ventricle presenting with hemifacial spasm Report of a case. Rev Neurol (Paris). 1999; 155:309–311. PMID: 10367329.
36. Sataloff RT. Embryology and anomalies of the facial nerve and their surgical implications. New York: Raven Press;1991.
37. Selky AK, Purvin VA. Hemifacial spasm. An unusual manifestation of idiopathic intracranial hypertension. J Neuroophthalmol. 1994; 14:196–198. PMID: 7881522.

38. Singh AK, Jain VK, Chhabra DK, Hongo K, Kobayashi S. Hemifacial spasm and cerebellopontine angle epidermoid : case report and review. Neurol Res. 1994; 16:321–323. PMID: 7984265.

39. Tan EK, Chan LL, Lim SH, Lim WE, Khoo JB, Tan KP. Role of magnetic resonance imaging and magnetic resonance angiography in patients with hemifacial spasm. Ann Acad Med Singapore. 1999; 28:169–173. PMID: 10497660.
40. Tan EK, Jankovic J. Psychogenic hemifacial spasm. J Neuropsychiatry Clin Neurosci. 2001; 13:380–384. PMID: 11514645.

41. Tan NC, Chan LL, Tan EK. Hemifacial spasm and involuntary facial movements. QJM. 2002; 95:493–500. PMID: 12145388.

42. van de Biezenbos JB, Horstink MW, van de Vlasakker CJ, van Engelen BG, van Eikema Hommes OR, Barkhof F. A case of bilateral alternating hemifacial spasms. Mov Disord. 1992; 7:68–70. PMID: 1557068.

43. Vermersch P, Petit H, Marion MH, Montagne B. Hemifacial spasm due to pontine infarction. J Neurol Neurosurg Psychiatry. 1991; 54:1018. PMID: 1800652.

44. Wang A, Jankovic J. Hemifacial spasm : clinical findings and treatment. Muscle Nerve. 1998; 21:1740–1747. PMID: 9843077.
45. Westra I, Drummond GT. Occult pontine glioma in a patient with hemifacial spasm. Can J Ophthalmol. 1991; 26:148–151. PMID: 2054726.
Fig. 1
Preoperative magnetic resonance angiography. Compression of the left facial nerve root exit zone by the anterior inferior cerebellar artery is observed.
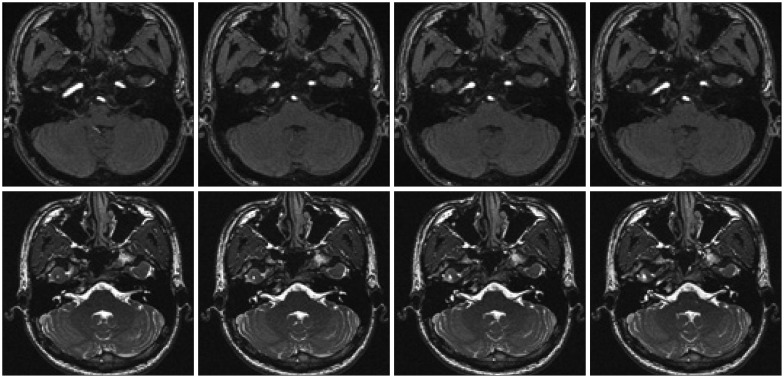
Fig. 2
Intraoperative findings. Penetration of the distal portion of the facial nerve root exit zone by the anterior inferior cerebellar artery is revealed (arrow). After longitudinally splitting the penetrated nerve, the compressing artery was moved away from the penetrating site and the decompression was secured by insertion of Teflon.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download