Abstract
Occasionally, unexpected neurological deficits occur after lumbar spinal surgery. We report a case of monoparesis after lumbar decompressive surgery. A 63-year-old man, who had undergone decompression of L4-5 for spinal stenosis 4 days previously in the other hospital, visted the emergency department with progressive weakness in the left leg and hypoesthesia below sensory level T7 on the right side. He had been cured of lung cancer with chemotherapy and radiation therapy 10 years previously, but detailed information of radiotherapy was not available. Whole spine magnetic resonance (MR) imaging showed fatty marrow change from T1 to T8, most likely due to previous irradiation. The T2-weighted MR image showed a high-signal T4-5 spinal cord lesion surrounded by a low signal rim, and the T1-weighted MR image showed focal high signal intensity with focal enhancement. The radiological diagnosis was vascular disorders with suspicious bleeding. Surgical removal was refused by the patient. With rehabilitation, the patient could walk independently without assistance 2 months later. Considering radiation induced change at thoracic vertebrae, vascular disorders may be induced by irradiation. If the spinal cord was previously irradiated, radiation induced vascular disorders needs to be considered.
Occasionally, unexpected neurological deficits occur after spinal surgery. If the patient complains of leg symptoms/back pain, and there is a well-correlated lesion in lumbar spinal magnetic resonance (MR) imaging, the lesion is usually regarded as the problem. However, if the presentation of symptoms/signs is not typical, concurrent lesion in the other spine level, which is reported between 5% and 28% of patients with lumbar spine stenosis, is suspected, and it is important not to make a misdiagnosis2,4,8,9). The patient's medical history also should be investigated thoroughly. We report a case of unexpected monoparesis after decompressive lumbar surgery for spinal stenosis. The monoparesis was caused by bleeding from probable vascular disorders such as cavernous malformation (CM) in the spinal cord, which should have been detected before the operation with a faithful physical examination and inquiry of the patient's medical history.
A 63-year-old man, who underwent decompressive laminectomy for spinal stenosis at L4-5 4 days previously in the other hospital, visited the emergency department with progressive weakness in his left leg and numbness below sensory level T7 on the right side and in the right leg. He had been cured of lung cancer with chemotherapy and conventional radiation therapy 10 years previously. Unfortunately, detailed information about the irradiation was not available, but usual protocol of radiotherapy was 60 Gy/30 fractions at that times. Before the operation, he had complained of numbness and pain in the right leg. Lumbar spine MR imaging showed spinal stenosis with spondylolisthesis at L4-5. Because the leg symptom was thought to be correlated with the lumbar stenosis, decompressive laminectomy was planned. The patient's medical record showed that motor power was normal in the lower extremities. Neither sensory nor deep tendon reflex abnormality was mentioned. Although subjective numbness on right side trunk were present, further imaging studies were not performed for the cervical and thoracic spine.
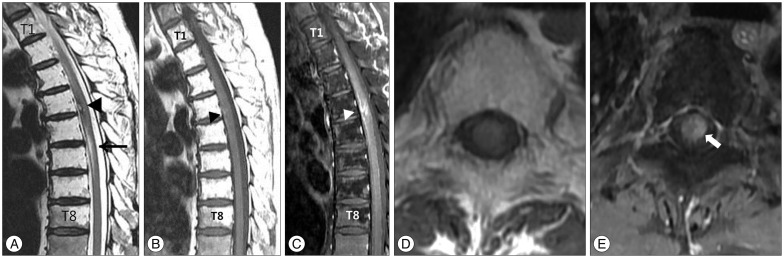
Decompressive laminectomy was uneventful, but left leg monoparesis (manual motor power grade II/V) suddenly developed 2 days after surgery. The patient's leg pain was not improved. Neurological examination demonstrated left leg weakness (manual motor power grades : hip flexion, I/V; knee extension, II/V; ankle dorsiflexion, II/V; ankle plantar flexion, II/V), decreased sensation to light touch and pain below right sensory level T7. Deep tendon reflex was slightly decreased. Whole spine MR imaging showed fatty marrow change from T1 to T8, most likely due to previous irradiation (Fig. 1). High signal intensity change in the T2-weighted MR image at T5-6 spinal cord showed normal diameter of the spinal cord without edema, and a sequela of previous irradiation was suspected (Fig. 1). The T2-weighted MR image showed another high-signal lesion at T4-5 spinal cord surrounded by a low signal rim (Fig. 1), and the T1-weighted MR image showed focal high signal intensity (Fig. 1) with irregular gadolinium enhancement. The radiological interpretations were : suspicious bleeding, myelopathy, probable CM, and fatty marrow change from T1 to T8. Considering past medical history and fatty marrow change at the vertebrae which seemed to be the field of irradiation, vascular disorders such as CM were suspected as sequel of irradiation. The patient's weakness was improved with rehabilitation. The patient could walk 100 m without assistance 2 months later, but hypoesthesia persisted in the right side of the trunk and the leg. The patient refused the removal of the probable CM.
We reported a case of monoparesis after common lumbar decompressive laminectomy probably due to lesion at the thoracic spine. Considering radiation induced change at thoracic vertebrae and spinal cord, vascular disorders such as CM may be induced by irradiation. Many hemorrhagic vascular disorders may have similar radiological findings and without pathological examination and MR image before radiotherapy, the diagnosis was not conclusive18). However, careful attention should have been paid to the history of irradiation to the spinal cord. Infrequently, patients have suffered from unexpected neurological deterioration after common lumbar spinal surgery, and concurrent tandem lesions in other levels of the spine were among the causes2,4,8,18). Careful physical examination and imaging studies were recommended in suspected cases to prevent catastrophe2,4,8). However, a false negative neurological examination was reported in 37% of patients with myelopathy, and suspicion with medical histories involving diseases such as cancer was also important6).
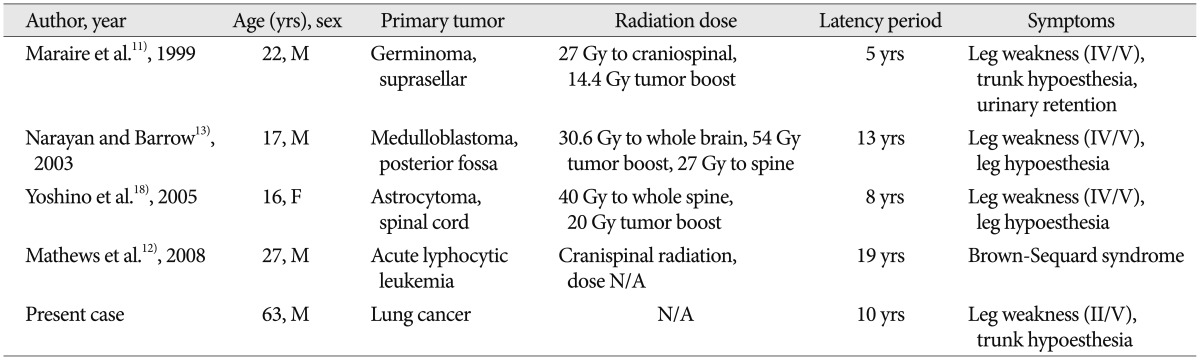
A cavernous malformationis is a blood vessel hamartoma that can affect any part of the central nervous system14,15,16,17,19). Spinal cord CMs represent 5-12% of all spinal vascular abnormalities19). These lesions are presumed to be congenital14,15,16,17), but radiation-induced CMs were reported after irradiation to the central nervous system, and the spinal cord was no exception11,13,14,17,18). There was two possible theories for the formation of cavernous malformation after radiation. First, the CM may have present, but it was undetectable on neuroimaging study and enlarged due to vascular insult by radiation. Second, the CM may have formed de novo in response to radiotherapy18). Radiation induced proliferation and dilation of the vascular endothelium with hyalinization and fibrinoid necrosis of blood vessel walls may induced cavernous malformation5,7,10). Four cases of de novo development of spinal CMs following spinal axis radiation have been previously reported. The first report was published in 1999 by Maraire et al.11); it involved a 22-year-old man who received 27 Gy of craniospinal radiation and a 14.4 Gy tumor boost after resection of suprasellar germinoma (Table 1). Five years later, a CM developed. The second case, reported by Narayan and Barrow13) in 2003, involved a 17-year-old boy who underwent radiation therapy (30.6 Gy to the whole brain, 54 Gy tumor boost, and 27 Gy to the entire spine) after resection of posterior fossa medulloblastoma. This patient developed a spinal cord CM 13 years after craniospinal irradiation. The third case, reported by Yoshino et al.18) in 2005, involved a 16-year-old girl who underwent neuraxis radiotherapy (40 Gy to the whole spine, 20 Gy tumor boost) after resection of pilocytic astrocytoma of the spinal cord. A spinal CM developed at the irradiation field 8 years later. The fourth case was reported by Mathews et al.12) in 2008. A 27-year-old man presented with acute Brown-Sequard syndrome. Craniospinal radiation (radiation dose was not describled) to treat acute lymphocytic leukemia 19 years before. MR image demonstrated CM with bleeding and the patient was managed with steroid medication. Two CM13,18) occurred after irradiation to the spinal cord after surgery for spinal intramedullary tumor and the others11,12) occurred after craniospinal irradiation. Their clinical presentation was motor weakness and decreased sensation. MR imaging revealed that the CMs were associated with cysts in 2 cases11,13,18). Pathological features of three cases11,13,18) demonstrated large, thin-walled blood vessels which were consistent with CM and vascular endothelial dilation and fibrinoid necrosis, which was typical of radiation-induced vascular change11,13,18). Radiotherapy induced CM at the spinal rootlets due to renal cell cancer (radiation dose was not available), Hodgkin's disease (40 Gy/20 fractions, 45 Gy/40 fractions), testicular seminoma (radiation dose was not available) at 24, 13, 26, and 47 years after radiotherapy, respectively3,10).
In the present case, the diagnosis was not conclusive without pathological confirmation. Although the result was obtained from brain, not cavernous malformation, but radiation-induced telangiectasia may present with varying amounts of hemorrhage and, occasionally, parenchymal hematomas, and may appear similar to cryptic vascular malformations on T2-weighted MR images5). To prove radiation induced CM we need to verify the following conditions; no lesion before radiation, latency period, the lesion within the radiation field and pathological examination1). Considering those criteria, there was not enough evidence to support the diagnosis of radiation induced CM in the present case.
This case is suggestive that showed the importance of faithful neurological examination and history taking. A radiation induced lesion may be suspected if neurologic symptoms/signs are atypical and the spinal cord was previously irradiated even for patients with common lumbar degenerative disease.
Acknowledgements
I appreciate professor Hak Jae Kim, M.D., Ph.D. for the critical comment regarding Radiation oncology.
This work was supported by Grant No. 03-2013-0330 from the Seoul National University Hospital Research Fund. The case report is approved by Institutional Review Board in Seoul National University Hospital (H 1403-063-564).
References
1. Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone : report of eleven cases. 1948. Cancer. 1998; 82:8–34. PMID: 9428476.
2. Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg. 1987; 66:842–849. PMID: 3572515.
3. Ducray F, Guillevin R, Psimaras D, Sanson M, Mokhtari K, Delanian S, et al. Postradiation lumbosacral radiculopathy with spinal root cavernomas mimicking carcinomatous meningitis. Neuro Oncol. 2008; 10:1035–1039. PMID: 18755918.

4. Epstein NE, Epstein JA, Carras R, Murthy VS, Hyman RA. Coexisting cervical and lumbar spinal stenosis : diagnosis and management. Neurosurgery. 1984; 15:489–496. PMID: 6493458.

5. Gaensler EH, Dillon WP, Edwards MS, Larson DA, Rosenau W, Wilson CB. Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology. 1994; 193:629–636. PMID: 7972799.

6. Glaser JA, Curé JK, Bailey KL, Morrow DL. Cervical spinal cord compression and the Hoffmann sign. Iowa Orthop J. 2001; 21:49–52. PMID: 11813951.
7. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain : a late effect predominantly in children. Cancer. 2002; 94:3285–3291. PMID: 12115362.

8. Hsieh CH, Huang TJ, Hsu RW. Tandem spinal stenosis : clinical diagnosis and surgical treatment. Changgeng Yi Xue Za Zhi. 1998; 21:429–435. PMID: 10074729.
9. Jung DY, Cho KT, Lee SC. Atypical guillain-barré syndrome misdiagnosed as lumbar spinal stenosis. J Korean Neurosurg Soc. 2013; 53:245–248. PMID: 23826482.

10. Labauge P, Lefloch A, Chapon F, Castelnovo G, Maubon A, Rigau V, et al. Postirradiation spinal root cavernoma. Eur Neurol. 2006; 56:256–257. PMID: 17077638.

11. Maraire JN, Abdulrauf SI, Berger S, Knisely J, Awad IA. De novo development of a cavernous malformation of the spinal cord following spinal axis radiation. Case report. J Neurosurg. 1999; 90(2 Suppl):234–238. PMID: 10199254.

12. Mathews MS, Peck WW, Brant-Zawadzki M. Brown-Séquard syndrome secondary to spontaneous bleed from postradiation cavernous angiomas. AJNR Am J Neuroradiol. 2008; 29:1989–1990. PMID: 18617590.

13. Narayan P, Barrow DL. Intramedullary spinal cavernous malformation following spinal irradiation. Case report and review of the literature. J Neurosurg. 2003; 98(1 Suppl):68–72. PMID: 12546391.

14. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006; 21:e4. PMID: 16859257.

15. Sure U, Freman S, Bozinov O, Benes L, Siegel AM, Bertalanffy H. Biological activity of adult cavernous malformations : a study of 56 patients. J Neurosurg. 2005; 102:342–347. PMID: 15739564.

16. Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. 2010; 29:E7. PMID: 20809765.

17. Wilson CB. Cryptic vascular malformations. Clin Neurosurg. 1992; 38:49–84. PMID: 1537199.
18. Yoshino M, Morita A, Shibahara J, Kirino T. Radiation-induced spinal cord cavernous malformation. Case report. J Neurosurg. 2005; 102(1 Suppl):101–104. PMID: 16206743.
19. Zevgaridis D, Medele RJ, Hamburger C, Steiger HJ, Reulen HJ. Cavernous haemangiomas of the spinal cord. A review of 117 cases. Acta Neurochir (Wien). 1999; 141:237–245. PMID: 10214479.

Fig. 1
Magnetic resonance imaging from the 63-year-old man with spinal cord cavernous malformation. A hyperintense intramedullary lesion (black arrowhead) is surrounded by hypointense rim by hemosiderin is seen at the T4-5 level on the T2-weighted sagittal magnetic resonance (MR) image (A). The lesion at T4-5 also shows hyperintensity on the T1-weighted MR image (black arrowhead), and which suggests recent hemorrhage (B). The lesions (arrowhead, C and arrow, E) showed irregular enhancement in T1-weighted sagittal and axial images (C, D, and E). Localized fatty marrow change from T1 to T8 vertebral bodies due to previous irradiation is also noted on the T1 and T2-weighted sagittal MR images (A and B). Another ill-defined high signal intensity change on the T2-weighted sagittal MR image at T5-6 spinal cord shows normal diameter of the spinal cord without edema, and a sequela of previous irradiation is suspected (black arrow, A).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download