Abstract
Objective
Removal of blood from subarachnoid space with a lumbar drainage (LD) may decrease development of cerebral vasospasm. We evaluated the effectiveness of a LD for a clinical vasospasm and outcomes after clipping of aneurysmal subarachnoid hemorrhage (SAH).
Methods
Between July 2008 and July 2013, 234 patients were included in this study. The LD group consisted of 126 patients, 108 patients in the non LD group. We investigated outcomes as follow : 1) clinical vasospasm, 2) angioplasty, 3) cerebral infarction, 4) Glasgow outcome scale (GOS) score at discharge, 5) GOS score at 6-month follow-up, and 6) mortality.
Results
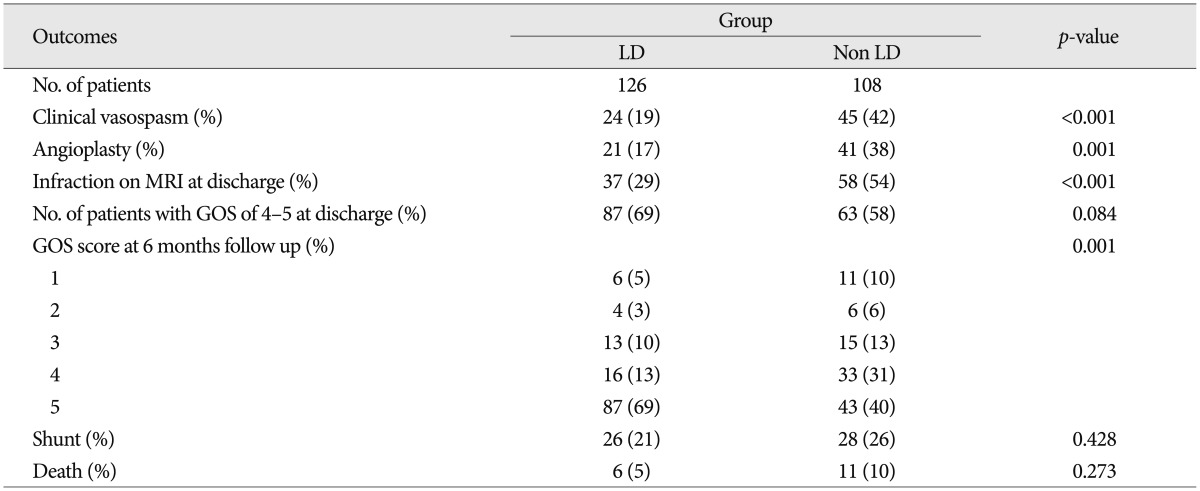
Clinical vasospasm occurred in 19% of the LD group and 42% of the non LD group (p<0.001). Angioplasty was performed in 17% of the LD group and 38% of the non LD group (p=0.001). Cerebral infarctions were detected in 29% and 54% of each group respectively (p<0.001). The proportion of GOS score 5 at 6 month follow-up in the LD group was 69%, and it was 58% in the non LD group (p=0.001). Mortality rate showed 5% and 10% in each group respectively. But, there was no difference in shunt between the two groups.
The management of patients with acutely ruptured intracranial aneurysms should achieve two major goals : 1) prevention of subsequent bleeding and 2) prevention and treatment of delayed ischemia or infarction due to vasospasm29). Early surgical clipping or endovascular coiling can prevent the subsequent aneurysm bleeding and can allow active management of vasospasm. In spite of marked development in the treatment of aneurysmal subarachnoid hemorrhage (SAH), the cerebral vasospasm still remains a significant cause of cerebral ischemia and neurologic deficits in patient with SAH. The prevalence of vasospasms have been reported 20-35% in aneurysmal SAH patients, although in those with a higher blood load, this may be as high as 40%2,18). Some authors reported that cerebral infarcts occurred in approximately 20% of patients and caused 13% of all death and disability after SAH2,18).
Although angiographic vasospasm may occur in up to 70% of patients, symptomatic vasospasms only occur in about 30% of patients10). Delayed ischemic neurologic deficits (DIND) may occur in about 50% of patients with angiographic vasospasm, which may lead to stroke or death despite maximal therapy26).
Although much has been elucidated regarding pathophysiology of the vasospasm, its exact pathophysiologic mechanism remains an incompletely solved problem. The presence of blood or its breakdown products within the subarachnoid space and cisterns is clearly associated with vasospasm9). So, thick SAH completely filling cistern has been shown to be an independent predictor of DIND2). Considerable clinical and experimental evidences have been reported that the volume and duration of subarachnoid blood clots are directly related to the development and severity of the cerebral vasospasm9,38). Moreover, the removal of blood clots and irrigation of the cisterns performed during surgery have been reported to reduce the risk of cerebral vasospasm15,20). Because hemolysis of blood is the primary inciting agent for vasospasms, it follows that strategies to facilitate the clearance of blood from the subarachnoid spaces shall decrease cerebral vasospasm. This strategy has been studied by a number of investigators7,13,15,19,22,27,32). A number of Japanese groups have advocated cisternal irrigation therapy with inflow and outflow catheters placed in the cranial subarachnoid spaces22,23,24,31,36).
Lumbar drainage (LD) is thought to be a simple and effective method to facilitate brain relaxation during aneurysmal SAH surgery, remove blood cell mass from the subarachnoid spaces, and decrease the incidence of vasospasm. Draining cerebrospinal fluid (CSF) from the lumbar subarachnoid space would be expected to promote circulation of clear, newly formed CSF from the cerebral ventricles through the subarachnoid spaces. Moreover, LD would also promote removal of the red cell mass from the intrathecal space, which represents the largest of all subarachnoid cisterns.
We have been using LD after SAH, and now report how this affects the incidence of vasospasm and improvement in clinical outcome in comparison to a group of patients whose SAH was managed with LD or no CSF drainage.
Between July 2008 and July 2013, 427 patients with aneurysmal SAH were hospitalized and treated in my hospital. 193 patients were excluded from this study for various reasons. We excluded patients whose neurological conditions at admission were too poor to manage the aneurysm properly and to allow clinical recognition of signs and symptoms those, who died within 7 days after rupture of aneurysm. The patients who were treated with endovascular coiling were also excluded. Because of minimal effect of LD for prevention of vasospasm, the patients with Fisher grade 1 were excluded. The patients who had received second operation like decompressive craniectomy or removal of intracerebral hematoma, were also excluded. We experienced 4 cases of non functioning drainage due to broken catheter, thick blood clot, insertion into epidural space. All non functioning drainage patients were excluded in this study.
Finally, we disqualified the patients who presented for surgical clipping 4 or more days after initial SAH. 234 patients were left as the patient population for this study.
At admission, we conducted brain computed tomography (CT) and neurologic examination and recorded Hunt and Hess grade, modified Fisher grade according to density of SAH on initial CT scan. We create a new modified Fisher grade 3+4 category for patients with both dense subarachnoid blood clot and intraventricular hemorrhages of 5 mL shown in Fig. 1.
All patients were treated with appropriate medication and procedure including intubation, mannitol, anticonvulsant, sedation and central vein catheterization for stabilization of systemic and neurologic condition.
All patients were managed according to our SAH management protocol. Our protocol included surgical clipping or coiling of ruptured aneurysms as soon as possible, at least within 24 hours after initial ictus, nimodipine administration, daily transcranial Doppler (TCD) examination, monitoring and correction of electrolyte and blood gas, control of intracranial pressure (ICP), pain management, and so on. The patients underwent CT or diffusion magnetic resonance imaging (MRI) and digital subtraction angiography (DSA) if vasospasm was suspected based on clinical symptoms and signs and TCD exam.
We confirmed the vasospasm with DSA, and then the interventional radiologist usually treated vasospasm with either repeated intra-arterial vasodilator infusion or balloon angioplasty.
From the start, treatment was provided in the form of intravenous nimodipine at a dose of 0.5 mg/h with gradual dose increments over the next 6 hours up to 2 mg/h in the absence of adverse hemodynamic effects, and was continued via the oral route at a dose of 60 mg every 4 hours.
We defined clinical vasospasm using the tirilizad trials18) as following : 1) newly developed neurological deficits such as confusion, disorientation, increased sleeping tendency, focal motor or speech deficits, and pupil reflex change; 2) no other cause of neurological deficits such as hyponatremia, hypoxia, infection, pulmonary edema, and drug toxicity; 3) negative findings on brain CT scan of secondary hemorrhage, cerebral edema, hydrocephalus; 4) evidence of vasospasm on serial TCD ultrasonography examinations. If the clinical symptoms and signs of vasospasm were suspected, we performed DSA immediately to confirm the vasospasm.
TCD exploration was carried out with 2 MHz ultrasound probe through the craniotomy site window in the case of a routine pterional approach and the temporal window in the case of supraorbital approach. TCD study was carried out in the first 24 hours and daily for 14 days following SAH. The studies were made by 3 physician assistants with extensive experience in ultrasonic Doppler examination. In both hemispheres, we recorded the mean velocity (MV) of the proximal middle cerebral artery (MCA). Also TCD exploration was made of the homolateral extracranial internal carotid artery (ex ICA) at submandibular level, with calculation of the Lindegaard index (LI) : MV MCA/MV ex ICA. The patients who presented over 150 m/s of MV or increases of more than 50 cm/s a day or an MV MCA/MV ex ICA ratio greater than 3 were classified as clinical vasospasm. DSA was carried out for accurate confirmation of arterial spasm and treatment of vasospasms.
We performed the LD if there were no evidences of the obstructive hydrocephalus and mass effect causing midline shift with CT scan. We randomly selected the patient on whom LD was performed at operation. LD was typically performed during clipping surgery. If the postoperative CT scan demonstrated no contraindication, the LD was opened. LD was continued throughout the vasospasm risk period (about 14 days after SAH). We used closed drainage system with infusion pump to drain the CSF slowly and continuously and the drain rate was 5-10 mL/h (Fig. 2). We examined CSF analysis every 3 days for detecting CSF infection.
The outcomes measure were 1) prevalence of clinical vasospasm, 2) incidence of angioplasty, 3) rate of cerebral infarction on MRI at discharge, 4) persisting neurological deficit at discharge by Glasgow outcome scale (GOS) score, 5) GOS score at 6-month follow-up, and 6) mortality rate.
Categorical variables are summarized as frequencies, percent-ages, and ranges. Tests of associations between the outcome criteria and the LD were performed using chi-square test or Fisher's exact test. We used Student t-test for continuous variables to compare two groups. Statistical significance was considered at probability values of less than 0.05. Subgroup specific analysis according to modified Fisher grade was also performed also. All statistical analyses were performed with commercially available software (version 22.0, SPSS Institute, Chicago, IL, USA).
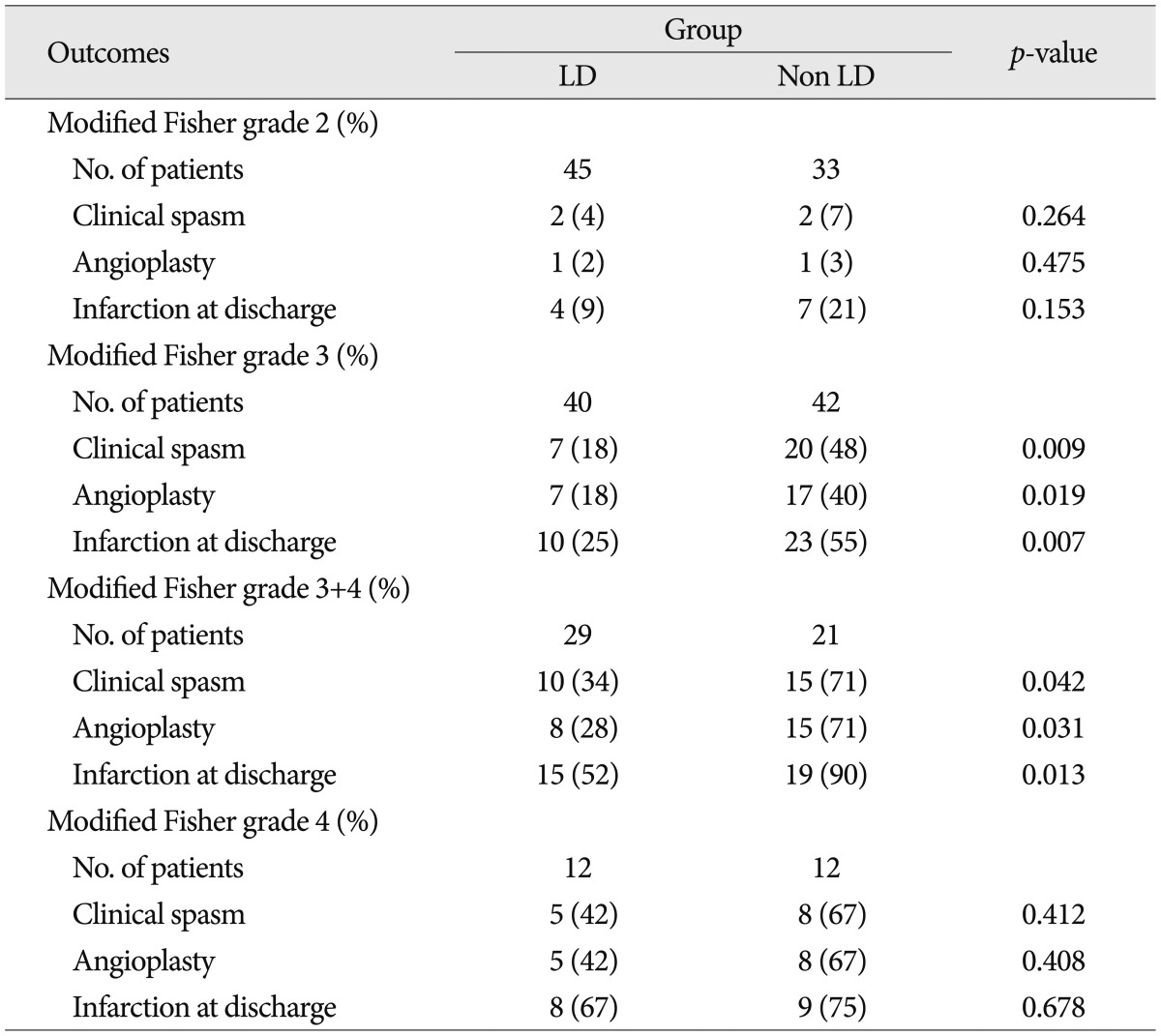
There were no significant differences in patient age, Hunt and Hess grade, aneurysm location and modified Fisher grade between the two groups (Table 1). Table 2 shows the favorable effects of LD on our numerous outcome criteria. The incidence of clinical vasospasm showed 19% in the LD group and 42% in the non LD group. Angioplasty for vasospasm treatment was performed in 17% of the LD group patients and 38% of the non LD group patients. On routine CT or MRI at discharge, the incidence of cerebral infarction was 29% in the LD group and 54% in the non LD group. The proportion of GOS score 5 at 6 month follow-up in the LD group was 69%, whereas 40% in the non LD group. LD group had 2 times fewer patients than that of the non LD group in poor GOS score of 1 and 2. Modified Fisher grade 3+4 category had the highest risk of clinical vasospasm (71%) in the non LD group and second high risk in the LD group (Table 3). Irrespective of LD, 33% of modified Fisher grade 3 patients and 50% of modified Fisher grade 3+4 patients suffered from clinical vasospasm (Table 3).
Mean CSF drainage was 174 mL/24 hours and the mean duration of drainage was 13.5 days (9-16.2 days).
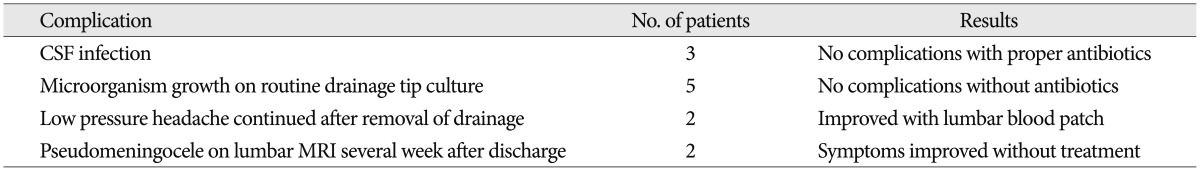
Complications associated with lumbar drains are presented in Table 4. Culture-positive meningitis developed in three patients of the LD group. After removal of drainage and treatment with proper antibiotics, these infections were resolved without permanent neurologic deficits. Five patients demonstrated microorganism growth on catheter tip culture without symptoms of meningitis, which was thought to be contaminated from skin. Two patients complained low intracranial pressure associated headache developed on several weeks after removal of the lumbar drain. Their headaches subsided after treatment with blood patch.
Despite advances in management of vasospasm, cerebral vasospasm is the still a significant cause of morbidity and mortality in treatment of aneurysmal SAH. The incidence and impact of vasospasm on clinical outcome after aneurysmal SAH has significantly decreased over past years. According to many studies for treatment after aneurysmal SAH, clinically significant vasospasm affected 5-13.5% of patients with permanent neurologic deficit and account for 33% of deaths and disabilities7,18). The current standard treatment for vasospasm after ruptured aneurysm surgery consists of triple-H (3H) therapy, calcium-channel blocker, and the endovascular angioplasty with papaverine or nimodipine injection. A number of studies have shown that 3H therapy and calcium channel blocker such as nimodipine reduced the patients with severe cerebral vasospasm18,28). Endovascular angioplasty and injection of intra-arterial chemical vasodilators enabled to treatment of vasospasm and thus improve their overall outcome3,6,8). But these current therapies can't prevent the occurrence of vasospasm in all patients, so cerebral vasospasm still contributes to poor outcome in approximately 10-40% of patients with dense SAH.
All patients were operated on and treated by one surgeon in the cerebrovascular center of our hospital with a consistent aneurysmal SAH management protocol. Although much has been elucidated regarding pathophysiology of vasospasm, the exact mechanisms are not completely understood. The blood in subarachnoid space or its breakdown products within the subarachnoid space is clearly associated with vasospasm2,15). Biochemical factors, such as oxygen free radicals, oxyhemoglobin, iron, intracellular adhesion molecule-1, endothelins, nitrous oxide, reduced form of nicotinamide-adenine dinucleotide phosphate oxidase, vascular endothelial growth factor, arachidonic acid, and protein kinase C from blood clots within subarachnoid space and cistern are known to be related to cerebral vasospasm4,16,17,21). Because hemolysis of blood is the primary inciting agent for vasospasm, there have been numerous clinical investigation that early clearance of subarachnoid blood clot or even massive subarachnoid irrigation would reduce the risk of vasospasm7,15,19,22,27,32). Many Japanese groups have applied massive irrigation therapy with catheters located in the subarachnoid spaces13,23,24,31,36), and this therapy is usually accompanied with daily head shaking and fibrinolytic therapy. But these therapies have not been accepted widespreadly, because the results were equivocal and there were risks of potential complication of cerebral hemorrhage and infection33,36).
It is impossible to clear completely blood clots in subarachnoid spaces and cisterns by surgery. The rationale of our use of lumbar CSF drainage in aneurysmal SAH is that it evacuates the large reservoir of bloody CSF from the spinal cistern, that it promotes CSF circulation from the ventricles through the subara-chnoid spaces, and that it also removes the biochemical substance to mediate vasospasm from subarachnoid space. Several reports have indicated that direct ventricular CSF drainage could reduce the incidence of vasospasm after aneurysmal SAH15,19). However, CSF drainage directly from the ventricles through extraventricular drainage (EVD) may disturb CSF circulation, and contribute to stasis of hemorrhage within the subarachnoid cisterns, so that ventricular drainage in those patients may actually add to the risk that cerebral vasospasm will develop. We excluded the patients with EVD in this study.
We routinely checked TCD parameter (MCA velocity, daily velocity trend, and LI) to detect vasospasm early from post SAH day 1 to day 14. MCA velocity above 200 cm/s may indicate moderate to severe vasospasm37). Other criterion for the possibility of development of severe vasospasm is increases of more than 50 cm/s per day5). It should be cautioned that velocity or increases in TCD velocity cannot distinguish vasospasm from cerebral hyperemia. So another criterion for detection of vasospasm was introduced. The extracranial and intracranial measurements can also be combined in a single ratio : vintracranial MCA/vextracranial ICA which is frequently called the LI25). LI>3 in patients with elevated MV in MCA offers high specificity (94-100%) in detecting vasospasm in MCA1,34). We used TCD parameters such as LI>3, increase of more than 50 cm/s a day, >150 cm/s of MCA velocity as vasospasm diagnostic criteria in this study. In my series, 3 patients among 30 clinical vasospasm patients showed no vasospasm on DSA in the LD group. LI were over 3 in two patients and one patient revealed that MV was 195 m/sec and increase of velocity was 55 m/sec a day. A technical error during TCD test was thought to be the cause of discordance. To reduce technical error, 3 physician assistant with at least 3 years experiences years could perform the TCD in my institute.
Cerebral infarction has been reported in 30% to 50% of patients after aneurysmal subarachnoid hemorrhage12,14). Naidech et al.30) reported that in 54% patients with SAH, radiographic cerebral infarction were detected on CT or MRI, and vasospasm was associated with a higher risk of cerebral infarction detection. Cerebral infarctions were detected 29% patients in the LD group and 54% in the non LD group. We only checked the infarction rate at discharge. We expect further study for investigation of many factors such as infarction site, volume, time, and so on which may influence on prognosis.
One of the advantages of LD is that it may lower the ICP at postoperative period. Although ICP was not specifically measured in this study, it is our impression that most patients with LD showed elevated opening CSF pressure and improvement of headache in the severity. Drainage of 5-20 mL of CSF through LD has been shown to approximately halve ICP in patients with aneurysmal SAH and those with brain injury35). There are a number of studies that lumbar drainage in aneurysmal SAH patients has benefit for lowering ICP and improvement in regional cerebral blood flow and oxygenation11,37). It is plausible that reduction of ICP may improve oxygenation and cerebral blood flow and thus reduce the prevalence of cerebral vasospasm.
Rebleeding of the ruptured aneurysm and neurological deterioration by cerebral herniation after insertion of a lumbar drainage have been well known to be the most serious complication. Because we examined CT scans before LD and all patients underwent insertion of LD catheter after being fully sedated with proper anesthesia, this dangerous problem was fortunately not encountered in our series. Two patients reported continued low pressure headaches but improved with epidural blood patch. We encountered lumbar insertion site epidural CSF collection in two patients' lumbar MRI during evaluation of their back pains which were developed in 2 weeks after removal of LD catheter. Their back pain was resolved spontaneously without any treatment. Coplin et al.3) reported that CSF infection after LD occurred at 4.2%, often appeared within 24 hours after insertion of LD catheter, happened most often with skin organisms, and CSF cell counts might not offer any additional useful information in diagnosing the complication. CSF infection secondary to lumbar drainage presented in 3 patients (3.7%) in my study, the organism was Staphylococcus aureus in all cases and was well controlled with proper antibiotics. There was no permanent morbidity associated with infection. We did not experience the complication of LD like spinal nerve root injury or tension pneumocephalus. To avoid complication and for the safe use of postoperative LD in patients with aneurysmal SAH, experienced team in my institute consisted of vascular neurosurgeon and neurosurgery nurse practitioner trained in the neurological critical care is fully occupied with strict attention to detail, close surveillance to the patient.
There are some limitations to our study. Selection bias is always a potential problem, although case assignment did have a randomizing effect. We did not distinguish the opening the laminar terminalis during clipping of surgery, which change the CSF pathway from 3rd ventricle to subarachnoid cistern directly and thus may affect the development of vasospasm.
In this study, clinical vasospasm and endovascular procedure to manage the vasospasm were marked reduced with LD after aneurysmal SAH surgery. Also, this method favorably influenced the GOS score at 6 month follow-up. Clearance of blood clots and its various derivatives from subarachnoid space and improvement of CSF circulation with LD were believed to be possible mechanisms to decrease incidence of cerebral vasospasm. There was no permanent morbidity and mortality related with LD. This report was only surgical series, we would expect investigation of vasospasm comparing surgical clipping series with endovascular coiling series. A randomized, prospective study will be able to overcome the limitations of our study.
References
1. Carrera E, Schmidt JM, Oddo M, Fernandez L, Claassen J, Seder D, et al. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009; 65:316–323. discussion 323-324. PMID: 19625911.

2. Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage : the Fisher scale revisited. Stroke. 2001; 32:2012–2020. PMID: 11546890.

3. Coplin WM, Avellino AM, Kim DK, Winn HR, Grady MS. Bacterial meningitis associated with lumbar drains : a retrospective cohort study. J Neurol Neurosurg Psychiatry. 1999; 67:468–473. PMID: 10486393.

4. Dietrich HH, Dacey RG Jr. Molecular keys to the problems of cerebral vasospasm. Neurosurgery. 2000; 46:517–530. PMID: 10719847.

5. Ekelund A, Säveland H, Romner B, Brandt L. Is transcranial Doppler sonography useful in detecting late cerebral ischaemia after aneurysmal subarachnoid haemorrhage? Br J Neurosurg. 1996; 10:19–25. PMID: 8672254.

6. Eskridge JM, McAuliffe W, Song JK, Deliganis AV, Newell DW, Lewis DH, et al. Balloon angioplasty for the treatment of vasospasm : results of first 50 cases. Neurosurgery. 1998; 42:510–516. discussion 516-517. PMID: 9526985.
7. Findlay JM, Kassell NF, Weir BK, Haley EC Jr, Kongable G, Germanson T, et al. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery. 1995; 37:168–176. discussion 177-178. PMID: 8587685.

8. Firlik KS, Kaufmann AM, Firlik AD, Jungreis CA, Yonas H. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1999; 51:66–74. PMID: 9952126.

9. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980; 6:1–9. PMID: 7354892.

10. Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage : an update. Ann Neurol. 1983; 14:599–608. PMID: 6651248.
11. Heuer GG, Smith MJ, Elliott JP, Winn HR, LeRoux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004; 101:408–416. PMID: 15352597.

12. Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage : clinicoanatomic correlations. Neurology. 1986; 36:329–333. PMID: 3951698.

13. Hoekema D, Schmidt RH, Ross I. Lumbar drainage for subarachnoid hemorrhage : technical considerations and safety analysis. Neurocrit Care. 2007; 7:3–9. PMID: 17624500.

14. Hoh BL, Curry WT Jr, Carter BS, Ogilvy CS. Computed tomographic demonstrated infarcts after surgical and endovascular treatment of aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2004; 146:1177–1183. PMID: 15349755.

15. Inagawa T, Kamiya K, Matsuda Y. Effect of continuous cisternal drainage on cerebral vasospasm. Acta Neurochir (Wien). 1991; 112:28–36. PMID: 1763681.

16. Juvela S. Plasma endothelin and big endothelin concentrations and serum endothelin-converting enzyme activity following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002; 97:1287–1293. PMID: 12507125.

17. Kamezaki T, Yanaka K, Nagase S, Fujita K, Kato N, Nose T. Increased levels of lipid peroxides as predictive of symptomatic vasospasm and poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002; 97:1302–1305. PMID: 12507127.

18. Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1 : Overall management results. J Neurosurg. 1990; 73:18–36. PMID: 2191090.
19. Kasuya H, Shimizu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage : a retrospective analysis of 108 patients. Neurosurgery. 1991; 28:56–59. PMID: 1994282.

20. Kawakami Y, Shimamura Y. Cisternal drainage after early operation of ruptured intracranial aneurysm. Neurosurgery. 1987; 20:8–14. PMID: 3808282.

21. Kim DE, Suh YS, Lee MS, Kim KY, Lee JH, Lee HS, et al. Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke. 2002; 33:2687–2691. PMID: 12411662.

22. Kodama N, Matsumoto M, Sasaki T, Konno Y, Sato T. Cisternal irrigation therapy with urokinase and ascorbic acid for prevention of vasospasm. Acta Neurochir Suppl. 2001; 77:171–174. PMID: 11563280.

23. Kodama N, Sasaki T, Kawakami M, Sato M, Asari J. Cisternal irrigation therapy with urokinase and ascorbic acid for prevention of vasospasm after aneurysmal subarachnoid hemorrhage. Outcome in 217 patients. Surg Neurol. 2000; 53:110–117. discussion 117-118. PMID: 10713187.
24. Konno Y, Sato T, Suzuki K, Matsumoto M, Sasaki T, Kodama N. Sequential changes of oxyhemoglobin in drained fluid of cisternal irrigation therapy--reference to the effect of ascorbic acid. Acta Neurochir Suppl. 2001; 77:167–169. PMID: 11563278.
25. Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien). 1989; 100:12–24. PMID: 2683600.

26. Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage : a population-based study in King County, Washington. Neurology. 1993; 43:712–718. PMID: 8469328.

27. Mizoi K, Yoshimoto T, Takahashi A, Fujiwara S, Koshu K, Sugawara T. Prospective study on the prevention of cerebral vasospasm by intrathecal fibrinolytic therapy with tissue-type plasminogen activator. J Neurosurg. 1993; 78:430–437. PMID: 8433145.

28. Mori K, Arai H, Nakajima K, Tajima A, Maeda M. Hemorheological and hemodynamic analysis of hypervolemic hemodilution therapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 1995; 26:1620–1626. PMID: 7660409.

29. Murayama Y, Malisch T, Guglielmi G, Mawad ME, Viñuela F, Duckwiler GR, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms : report on 69 cases. J Neurosurg. 1997; 87:830–835. PMID: 9384391.

30. Naidech AM, Bendok BR, Bassin SL, Bernstein RA, Batjer HH, Bleck TP. Classification of cerebral infarction after subarachnoid hemorrhage impacts outcome. Neurosurgery. 2009; 64:1052–1057. discussion 1057-1058. PMID: 19487883.

31. Nakagomi T, Takagi K, Narita K, Nagashima H, Tamura A. Cisternal washing therapy for the prevention of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2001; 77:161–165. PMID: 11563277.

32. Ohman J, Servo A, Heiskanen O. Effect of intrathecal fibrinolytic therapy on clot lysis and vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1991; 75:197–201. PMID: 1906535.

33. Seiler RW, Binggeli R. Is cerebral vasospasm still a clinical problem? Acta Neurochir Suppl. 2001; 77:1–4. PMID: 11563263.

34. Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, et al. Assessment : transcranial Doppler ultrasonography : report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004; 62:1468–1481. PMID: 15136667.

35. Suarez JI, Qureshi AI, Yahia AB, Parekh PD, Tamargo RJ, Williams MA, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage : evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002; 30:1348–1355. PMID: 12072693.

36. Treggiari-Venzi MM, Suter PM, Romand JA. Review of medical prevention of vasospasm after aneurysmal subarachnoid hemorrhage : a problem of neurointensive care. Neurosurgery. 2001; 48:249–261. discussion 261-262. PMID: 11220367.

37. Tuettenberg J, Czabanka M, Horn P, Woitzik J, Barth M, Thomé C, et al. Clinical evaluation of the safety and efficacy of lumbar cerebrospinal fluid drainage for the treatment of refractory increased intracranial pressure. J Neurosurg. 2009; 110:1200–1208. PMID: 19249925.

38. Zabramski JM, Spetzler RF, Bonstelle C. Chronic cerebral vasospasm : effect of volume and timing of hemorrhage in a canine model. Neurosurgery. 1986; 18:1–6. PMID: 3945370.

Fig. 1
Non-contrast axial CT scan showing the modified Fisher grade 3+4. A : CT scan shows dense subarachnoid hemorrhage in basal cistern and small amount of intraventricular hemorrhage in both lateral ventricle. B : Non-contrast CT scan demonstrates thick subarachnoid hemorrhage in basal cistern and small amount of intraventricular hemorrhage in 3rd ventricle and lateral ventricle.
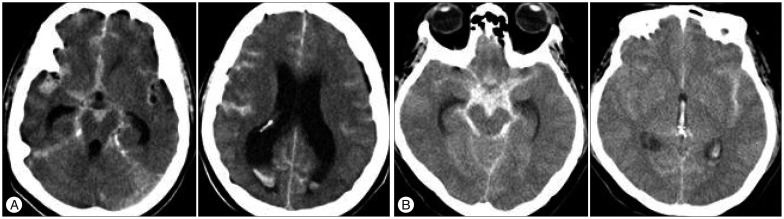
Fig. 2
Photograph shows closed lumbar drainage kit composed infusion pump and closed drainage bag. We performed lumbar CSF drainage slowly and continuously with drainage rate 5 to 10 mL/hour. CSF : cerebrospinal fluid.
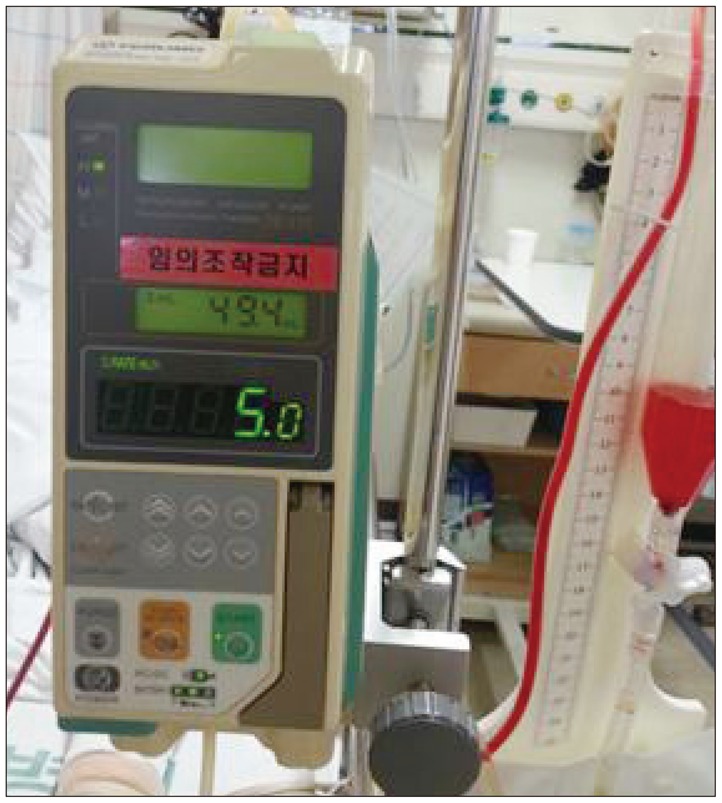
Table 1
Baseline characteristics of the lumbar drain and non-lumbar drain groups in 234 patients with aneurysmal subarachnoid hemorrhage underwent surgical clipping
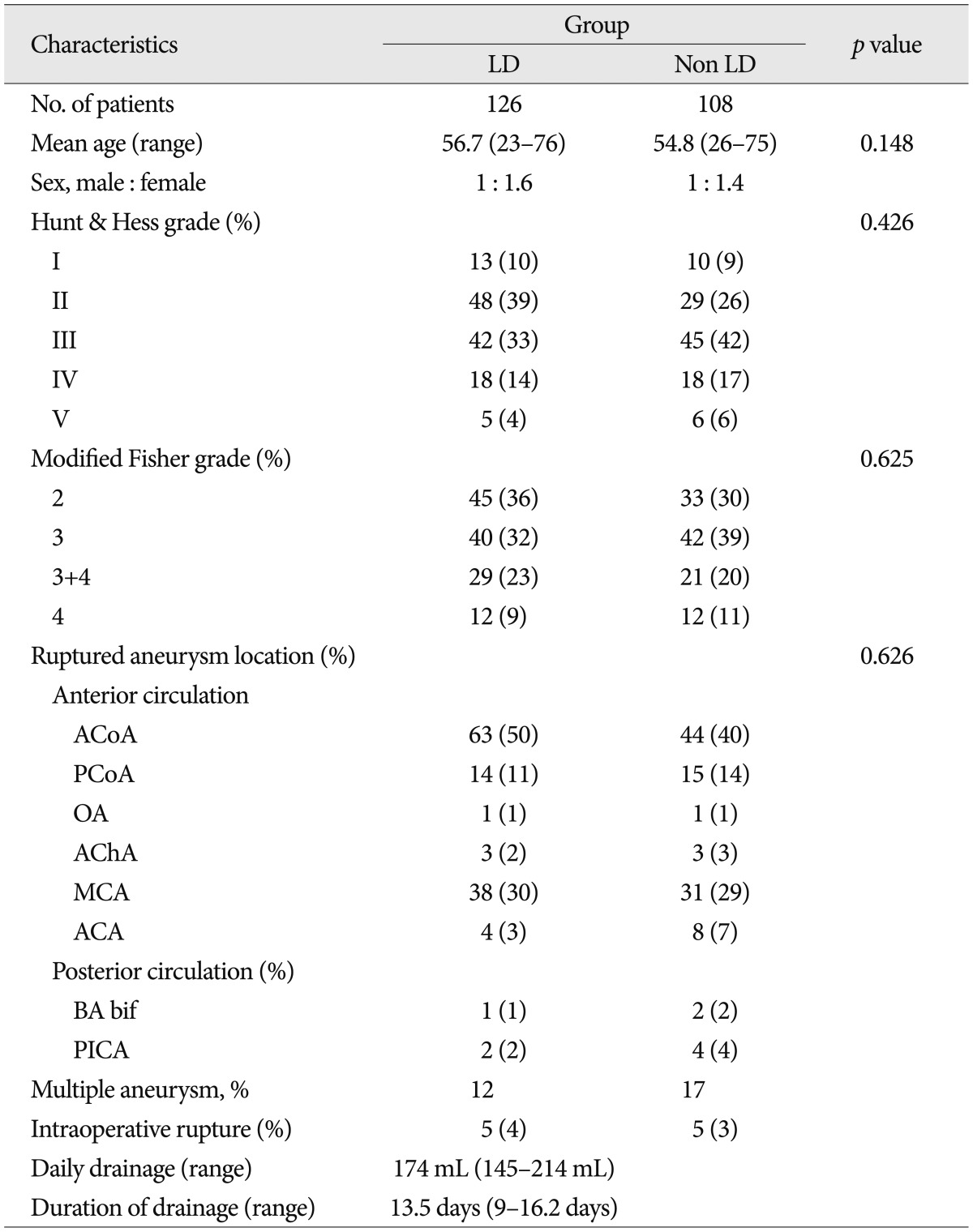
LD : lumbar drainage, ACoA : anterior communicating artery, PCoA : posterior communicating artery, OA : ophthalmic artery, AChA : anterior choroidal artery, MCA : middle cerebral artery, ACA : anterior cerebral artery distal to ACoA, BA bif : basilar artery bifurcation, PICA : posterior inferior cerebellar artery




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download