Abstract
Objective
Neuroblastoma is one of common childhood tumors. Although its mortality is very high, there is no effective treatment yet. The aim of this project is to evaluate cytotoxic effects of melatonin (MLT) an endogen hormone and 13-cis retinoic acid (13-cis-RA) also named as isotretinoin an analogue of vitamin A on neuroblastoma SH-SY5Y cell line.
Methods
In this study, SH-SY5Y cell line was used. After cell culture, the cells were exposed to different doses of MLT and 13-cis-RA. 24 and 48 hours later. While the viabilities was estimated with MTT cell viability assay test, apoptotic indexes were calculated after staining with TUNEL based apoptosis kit.
Results
It was observed that MLT has very effective cytotoxic potential than 13-cis-RA on neuroblastoma cell line. At the same time, when MLT and 13-cis-RA were combined, this effect was potentiated. On the other hand, it was found that the effect of 13-cis-RA individually on neuroblastoma cells was very slight.
Neuroblastoma is one of the commonly encountered tumors of the childhood with higher prevalence before age 10. The etiology of neuroblastoma is not clear because of its diverse manifestation characteristics. Although it is possible to effectively cure this disease during the first year of life when diagnosed prior to metastatic involvement, the mortality rate is very high5). It is well-known that melatonin (MLT) is an important hormone involved in circadian rhythm. Beside this, it has also been indicated that MLT has both neuroprotective and antioxidant effects1,11). In a study in which neuroprotective effect was investigated, it has been revealed that MLT exerts its protective effect on neurons by inhibiting the neo-regulation of microtubule formation in neuron cells1). It has also been reported that MLT has protective effect in some other conditions including lead-induced neurotoxicity16), calcyculin-A induced neurofilament deficiency10), hydrogen peroxide mediated neuronal loss2), cobalt mediated oxidative stress and neurotoxicity12), amphetamine induced neurotoxicity8).
13-cis retinoic acid (13-cis-RA) is a derivative of vitamin A and it is used in many fields of medical therapy, especially in dermatological treatment modalities. However, as 13-cis-RA is an oil-soluble substance and it is somewhat hardly eliminated from the body, insufficient or excessive intake of 13-cis-RA may lead to various clinical manifestations including mainly cerebral disorders. Neuroblastoma is also in the list of diseases against which 13-cis-RA treatment is being studied. In an in vivo experimental animal study conducted with vitamin A analogues, it has been reported that vitamin A derivatives have prominently decreased the tumoral mass and this has led to a controversy that if it could also be used in humans in a therapeutic manner13). But, there is no adequate number of studies about the place of 13-cis-RA in neuroblastoma treatment.
The aim of this preliminary research study is to investigate the cytotoxic effects of MLT and 13-cis-RA on SH-SY5Y cell line in both combined and stand-alone administration. There is no study about the possible effect of the combined treatment of 13-cis-RA and MLT, although there is plenty of studies which evaluate these two substances solely and this fact constitutes one of the specific aspects of our current study.
The SH-SY5Y human neuroblastoma cell line was obtained from Ege University Science and Technology Center (EBILTEM) and placed into 25 cm2 tissue culture flasks and was grown in monolayer culture in 85% Dulbecco's modified Eagle's medium (Biological Industries, Israel) supplemented with 15% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 units/mL penicillin, 100 µg/mL streptomycin (Biological Industries, Kibbutz Beit Haemek, Israel). Cultures were maintained in a 5% carbon dioxide/95% air-humidified incubator. Passaging and seeding was done by detaching the cell monolayers using 0.05% tripsin/ethylenediaminetetra acetic acid solution (Biological Industries, Kibbutz Beit Haemek, Israel). Trypan blue (Sigma-Aldrich, St. Louis, MO, USA) was used to ensure accurate cell count and measure the percentage of viability of the cell population. Following trypan blue assay, SH-SY5Y cells were plated in 96-well plates containing 200 µL culture medium at concentration of 5000 cells/well with ≥90% viability for MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay). On the other hand the cells were also grown on 6-well plates at a density of 5×105 for TUNEL assay.
MLT (N-acetyl-5-methoxytryptamine), 13-cis-RA and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Freshly prepared MLT was diluted in filter sterilized absolute alcohol and 13-cis-RA was diluted in dimetylsulfoxide (DMSO). They were further diluted in medium without serum. The final concentration of ethanol and DMSO was ≥0.5 in cell cultures.
Cellular viability was assessed with MTT assay as described previously. Cells were plated in 96-well plates containing 200 µL culture medium at concentration of 5000 cells/well with ≥90% viability for MTT. Following cell attachment (24 h), various concentrations and combinations of MLT and 13-cis-RA were applied (200-µL volume) to the wells to get the final concentration of 0.25, 0.5, 1, and 2 mM for MLT and 1, 5, 10 µM for 13-cis-RA combined and stand-alone. Thus the experiments were performed on different groups as follows; MLT alone, 13-cis-RA alone, MLT+(13-cis-RA), alcohol and DMSO solvent control, with n=5 in every experimental group. Cultures without drugs were studied as negative control groups. Stock MTT was prepared at 5 mg/mL in phosphate-buffered saline (PBS) filtered and kept in dark at 4℃. The cells were incubated for an additional 24 hours (24 h group) and 48 hours (48 h group). After the administration of drugs 20 µL MTT were added to microculture wells (total volume 220 µL). After 4 h incubation at 37℃ in dark, the unreactive supernatants in the well were discarded, and 100 µL of DMSO was added to solubilize the MTT-formazan product. The well plate was thoroughly mixed well and incubated additional 1 h in 37℃ in dark. Absorbance at 630 nm was measured with a microplate enzyme-linked immunosorbent assay reader (Trinity Biotech Captia, Co Wicklow, Ireland). Mean absorbance was calculated for the control wells and for each test wells. All procedure was repeated three times and mean values were calculated and used for statistical analysis.
The cells incubated with drugs for 24 and 48 hours and grown on 6-well plates were stained with commercially TUNEL based apoptosis detection kit (MK-500 Takara, Takara Bio Inc., Japan). The cells were trypsinized, centrifuged, resuspended, and fixed with 4% formaldehyde in PBS and then gently centrifuged again and the pellet was resuspended with 80% ethanole. Before immunohistochemical stain was begun, the suspended cells were smeared on poly-L-lysine coated slides and after the cells adhered to slides they were stained with TUNEL based apoptosis kit in order to manufacturer protocol.
The stained cells in the slides were counted in different 6 areas under ×20 objective magnification and the number of apoptotic brown in color cells and nonapoptotic green in color cells were summed and apoptotic index were estimated. For counting, NIS-Elements Imaging Software (Nikon, Japan) was used.
In the evaluation of viabilities by MTT of human SH-SY5Y cells exposed to different doses of MLT (0.25, 0.5, 1, 2 mM) we found that the viabilities of the cells decreased linearly parallel to increasing doses. At the same time, the viabilities of these cells exposed to different doses of 13-cis-RA (1, 5, 10 µM) were also decreased linearly parallel to increasing doses. But the decreasing of viabilities in MLT applied cells was much more than 13-cis-RA which were 73.1% vs. 30% for 24 hours and 62.6% vs. 34.2% for 48 hours respectively (Fig. 1A).
On the other hand, in the evaluation of apoptotic index of the cells detected by TUNEL based analysis we also found that the apoptotic index was decreased linearly parallel to increasing doses as in MTT assay (Fig. 2). Again, the apoptotic index in MLT applied cells was much more than 13-cis-RA which were 58.53% vs. 6.57% for 24 hours and 52.5% vs. 50.6% for 48 hours respectively (Fig. 1B).
Neuroblastoma is a widespread and malignant childhood cerebral tumoral disease. It is mainly treated with surgical intervention. Beside this, it has been indicated that various drugs have decreased the tumoral mass via different mechanisms and thereby increased the mean survival duration. One of these drugs is MLT, which is also an endogenous hormone. MLT exhibits its protective effect by decreasing the mean lipid peroxidase level and by increasing the antioxidant activity11). MLT exerts its antioxidant effect via tyrosine hydroxylase enzyme7). MLT has also inductive effect on neuronal differentiation, beside its antioxidant and neuroprotective effects9). On the other hand, it is known that melatonyl valproate has more robust neuroprotective effect than MLT20). MLT is considered as a beneficial therapeutic agent, as it can easily pass blood-brain barrier5). In a nitric-oxide induced neuronal apoptosis model study, it has been indicated that MLT has inhibitor effect on apoptosis by preventing bad translocation from cytoplasm to mitochondrion3). In numerous experimental studies, it has been reported that MLT has ameliorating effect on general clinical manifestation in diverse malignant or progressive diseases such as neuroblastoma and Parkinson's disease, beside its protective effect on healthy neuron cells. For example, it has been indicated that MLT decreased the α-synnuclein expression levels via direct or indirect ways in postnatal rats, which plays an important role in Parkinson's disease15). In transgenic mice with Alzheimer's disease model, it has also been revealed that MLT slowed down the progression of disease via the mitochondrial steps and thereby contributed to the acquisition of cognitive functions4). In other studies conducted with neuroblastoma cell lines, MLT prevented neurotoxicity by inhibiting the deleterious effect of methamphetamine, which is a dangerous member of amphetamine class of psychoactive drugs known to lead massive neuronal cell death in brain17). In another study, it has been reported that MLT has declined the cell death rates by suppressing the oxidative stress occurred in neuroblastoma cells following methamphetamine exposure21). In other conducted studies, it has been suggested that some different combinations of MLT with other agents could increase the effectiveness of MLT. For instance, it has been indicated that carvedilol combination of MLT can slow down the progression of many neurodegenerative diseases19) and combined galantamine usage has synergistically favorable effect on oxidative stress in neuroblastoma cells14). In numerous studies conducted on neurotoxicity, the protective effect of MLT has been clearly proved. For example, it has been indicated that MLT decreased the neurotoxic effect of nickel by suppressing oxidative stress and increasing mitochondrial function22). Similarly, the effectiveness of MLT has also been shown in lead-induced neurotoxicity16), neuronal cell death caused by hydrogen peroxide2) and cobalt-mediated oxidative stress and neurotoxicity12).
On the other hand, in some studies conducted on neuroblastoma cell lines, it has been reported that 13-cis-RA and its derivatives have induced differentiation in neuroblastoma cells over HASH1 gene expression6). Also in another study, it has been found that 13-cis-RA yielded favorable outcome by increasing apoptosis in CHP134 neuroblastoma cell line18). However, the number of related studies is still very low in the literature. In our study, we have observed that both MLT and 13-cis-RA have apoptosis-induced lethal effect on SIMA neuroblastoma cell line. However, this effect is more prominent in MLT and 13-cis-RA-induced apoptosis levels remained very low especially during the first 24 hours. At 48th hour, this activity has continued significantly on behalf of MLT. Interestingly, the prominently higher cytotoxic effect of combined 13-cis-RA and MLT treatment than the effectiveness of 13-cis-RA when administered alone suggests that there is a positive interaction between these two substances. Beside this, increment of apoptotic cell death rates and decrease in viability virtually in whole groups were in context of time and dose dependent manner.
Results gathered from the current study have indicated that MLT exhibited neurotoxic effect on SH-SY5Y neuroblastoma cell line and this effect was potentiated by 13-cis-RA. Thus, administration of these agents in treatment of patients with neuroblastoma may contribute to get favorable outcomes. However, as this study was planned and conducted under in vitro conditions with preliminary research approach, we believe that further and more comprehensive in vivo studies in which a clearly determined dose range and a suitable treatment protocol which would be prepared according to the data established from this study can give rise to more detailed and beneficial outcomes in this field in the future.
Acknowledgements
This study were financed by Afyon Kocatepe University Scientific Research Commission (09-TIP-20).
References
1. Benitez-King G, Túnez I, Bellon A, Ortíz GG, Antón-Tay F. Melatonin prevents cytoskeletal alterations and oxidative stress induced by okadaic acid in N1E-115 cells. Exp Neurol. 2003; 182:151–159. PMID: 12821385.

2. Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res. 2006; 41:116–123. PMID: 16879316.

3. Choi SI, Joo SS, Yoo YM. Melatonin prevents nitric oxide-induced apoptosis by increasing the interaction between 14-3-3beta and p-Bad in SK-N-MC cells. J Pineal Res. 2008; 44:95–100. PMID: 18078454.
4. Dragicevic N, Copes N, O'Neal-Moffitt G, Jin J, Buzzeo R, Mamcarz M, et al. Melatonin treatment restores mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res. 2011; 51:75–86. PMID: 21355879.

5. García-Santos G, Antolín I, Herrera F, Martín V, Rodriguez-Blanco J, del Pilar Carrera M, et al. Melatonin induces apoptosis in human neuroblastoma cancer cells. J Pineal Res. 2006; 41:130–135. PMID: 16879318.

6. Ichimiya S, Nimura Y, Seki N, Ozaki T, Nagase T, Nakagawara A. Downregulation of hASH1 is associated with the retinoic acid-induced differentiation of human neuroblastoma cell lines. Med Pediatr Oncol. 2001; 36:132–134. PMID: 11464865.

7. Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Melatonin inhibits amphetamine-induced increase in alpha-synuclein and decrease in phosphorylated tyrosine hydroxylase in SK-N-SH cells. Neurosci Lett. 2008; 436:309–313. PMID: 18406059.

8. Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Melatonin protects SK-N-SH neuroblastoma cells from amphetamine-induced neurotoxicity. J Pineal Res. 2007; 43:65–73. PMID: 17614837.

9. Lahiri DK, Ghosh C. Interactions between melatonin, reactive oxygen species, and nitric oxide. Ann N Y Acad Sci. 1999; 893:325–330. PMID: 10672259.

10. Li SP, Deng YQ, Wang XC, Wang YP, Wang JZ. Melatonin protects SH-SY5Y neuroblastoma cells from calyculin A-induced neurofilament impairment and neurotoxicity. J Pineal Res. 2004; 36:186–191. PMID: 15009509.

11. Montilla-López P, Muñoz-Agueda MC, Feijóo López M, Muñoz-Castañeda JR, Bujalance-Arenas I, Túnez-Fiñana I. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer's disease induced by okadaic acid in neuroblastoma cells. Eur J Pharmacol. 2002; 451:237–243. PMID: 12242084.

12. Olivieri G, Hess C, Savaskan E, Ly C, Meier F, Baysang G, et al. Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased beta-amyloid secretion. J Pineal Res. 2001; 31:320–325. PMID: 11703561.

13. Ponthan F, Borgström P, Hassan M, Wassberg E, Redfern CP, Kogner P. The vitamin A analogues: 13-cis retinoic acid, 9-cis retinoic acid, and Ro 13-6307 inhibit neuroblastoma tumour growth in vivo. Med Pediatr Oncol. 2001; 36:127–131. PMID: 11464864.

14. Romero A, Egea J, García AG, López MG. Synergistic neuroprotective effect of combined low concentrations of galantamine and melatonin against oxidative stress in SH-SY5Y neuroblastoma cells. J Pineal Res. 2010; 49:141–148. PMID: 20536682.

15. Sae-Ung K, Uéda K, Govitrapong P, Phansuwan-Pujito P. Melatonin reduces the expression of alpha-synuclein in the dopamine containing neuronal regions of amphetamine-treated postnatal rats. J Pineal Res. 2012; 52:128–137. PMID: 21851386.

16. Suresh C, Dennis AO, Heinz J, Vemuri MC, Chetty CS. Melatonin protection against lead-induced changes in human neuroblastoma cell cultures. Int J Toxicol. 2006; 25:459–464. PMID: 17132604.

17. Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells. J Pineal Res. 2010; 48:94–101. PMID: 20050990.

18. Takada N, Isogai E, Kawamoto T, Nakanishi H, Todo S, Nakagawara A. Retinoic acid-induced apoptosis of the CHP134 neuroblastoma cell line is associated with nuclear accumulation of p53 and is rescued by the GDNF/Ret signal. Med Pediatr Oncol. 2001; 36:122–126. PMID: 11464863.

19. Tasset I, Espínola C, Medina FJ, Feijóo M, Ruiz C, Moreno E, et al. Neuroprotective effect of carvedilol and melatonin on 3-nitropropionic acid-induced neurotoxicity in neuroblastoma. J Physiol Biochem. 2009; 65:291–296. PMID: 20119823.

20. Wang XC, Zhang YC, Chatterjie N, Grundke-Iqbal I, Iqbal K, Wang JZ. Effect of melatonin and melatonylvalpromide on beta-amyloid and neurofilaments in N2a cells. Neurochem Res. 2008; 33:1138–1144. PMID: 18231852.

21. Wisessmith W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells. J Pineal Res. 2009; 46:433–440. PMID: 19386024.

22. Xu SC, He MD, Lu YH, Li L, Zhong M, Zhang YW, et al. Nickel exposure induces oxidative damage to mitochondrial DNA in Neuro2a cells: the neuroprotective roles of melatonin. J Pineal Res. 2011; 51:426–433. PMID: 21797922.

Fig. 1
A: The viability rates in SH-SY5Y neuroblastoma cell line at the end of 24th and 48th hours (%) (MTT viability assay). B: The apoptotic index in SH-SY5Y neuroblastoma cell line at the end of 24th and 48th hours (%) (TUNEL based apoptosis detection). MTT: microculture tetrazolium, DMSO: dimetylsulfoxide, MLT: melatonin, RA: retinoic acid.
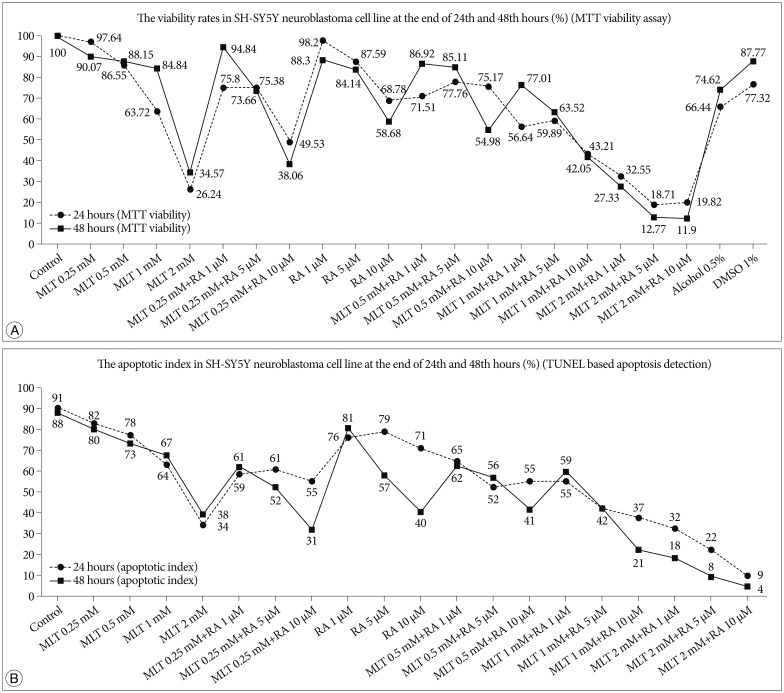
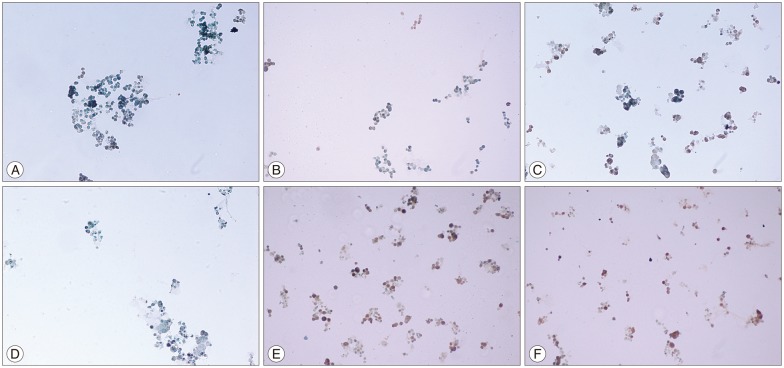
Fig. 2
A: The immunohistochemically stained SH-SY5Y neuroblastoma cells exposure to 13-cis-RA 1 µM (TdtTUNEL based apoptosis detection kit, MK500, Takara). The cells green in color are non-apoptotic and brown in color are apoptotic (×200). B: The immunohistochemically stained SH-SY5Y neuroblastoma cells exposure to 13-cis-RA 10 µM (TdtTUNEL based apoptosis detection kit, MK500, Takara). The cells green in color are non-apoptotic and brown in color are apoptotic (×200). C: The immunohistochemically stained SH-SY5Y neuroblastoma cells exposure to MLT 2 mM (TdtTUNEL based apoptosis detection kit, MK500, Takara). The cells green in color are non-apoptotic and brown in color are apoptotic (×200). D: The immunohistochemically stained SH-SY5Y neuroblastoma cells exposure to MLT 0.5 mM+13-cis-RA 1 µM (TdtTUNEL based apoptosis detection kit, MK500, Takara). The cells green in color are non-apoptotic and brown in color are apoptotic (×200). E: The immunohistochemically stained SH-SY5Y neuroblastoma cells exposure to MLT 1 mM+13-cis-RA 5 µM (TdtTUNEL based apoptosis detection kit, MK500, Takara). The cells green in color are non-apoptotic and brown in color are apoptotic (×200). F: The immunohistochemically stained SH-SY5Y neuroblastoma cells exposure to MLT 2 mM+13-cis-RA 10 µM (TdtTUNEL based apoptosis detection kit, MK500, Takara). The cells green in color are non-apoptotic and brown in color are apoptotic (×200). MLT: melatonin, RA: retinoic acid.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download