Abstract
Chorea-acanthocytosis (ChAc) is a rare hereditary disorder characterized by involuntary choreiform movements and erythrocytic acanthocytosis. Pharmacotherapy for control of involuntary movements has generally been of limited benefit. Deep brain stimulation (DBS) has recently been used for treatment of some refractory cases of ChAc. We report here on the effect of bilateral high-frequency DBS of globus pallidus interna in a patient with ChAc.
Chorea-acanthocytosis (ChAc) is a rare autosomal recessive neurodegenerative disorder characterized by generalized chorea, orofaciolingual dyskinesia with dysphagia and dysarthria, muscle wasting, hyporeflexia, and behavioral disturbance6). ChAc can be diagnosed by Western blot identification of chorein or sequencing of the VPS13 gene4,11). Effective management of ChAc has long been a significant challenge. Medical treatment is mostly ineffective and deep brain stimulation (DBS) has recently been attempted for management of ChAc. We report here on a genetically confirmed case of ChAc in a patient who showed significant improvement after bilateral DBS of the globus pallidus interna (GPi). We also provide a review of the literature regarding the treatment outcome of this rare condition.
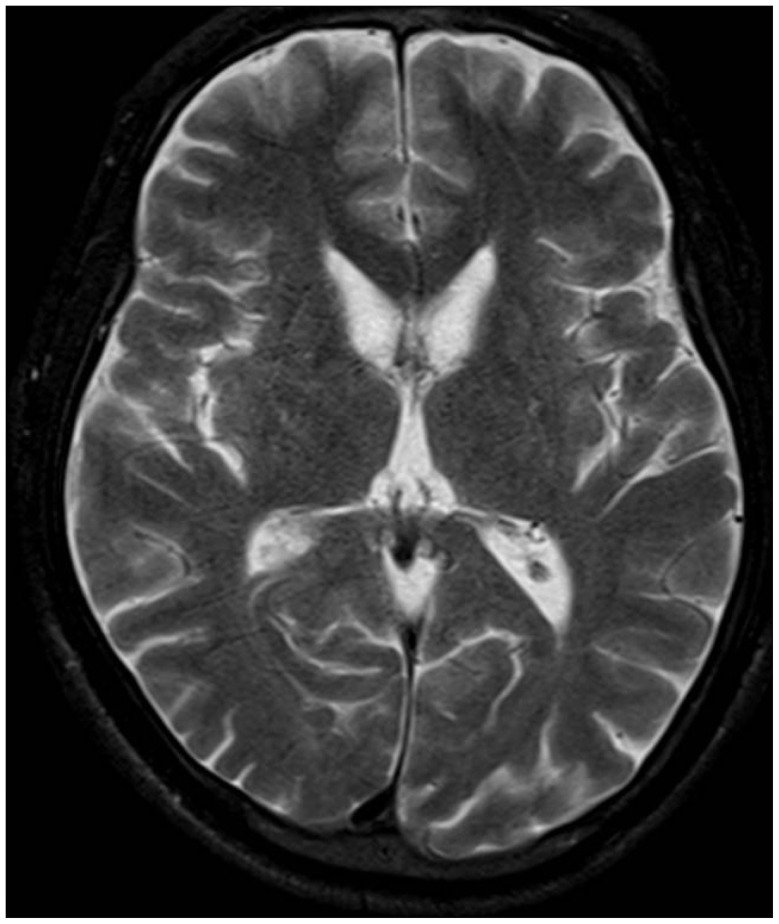
A 36-year-old man presented with exacerbation of slurred speech, orofaciolingual dyskinesia, tongue and lip biting, choreiform movements of the head and neck, and gait disturbance during the course of three years. There was no family history of similar neurological disorders. On examination, we observed rapid bending of the neck and trunk, alternating lateral flexion of the trunk when walking, resulting in frequent falls. Muscle power and muscle bulk, and sensory function were intact. The patient did not show any psychiatric disorder during the psychiatric interview. Mini-mental status examination (MMSE) score was 30 and intelligence quotient was 85. Laboratory tests, including serum creatine kinase, iron, ferritin, lactate, lipids, and lipid electrophoresis were unremarkable. Results of nerve conduction study, electromyography, and electroencephalography showed no abnormal findings. Cardiomyopathy, cardiac arrhythmia, and hepatosplenomegaly were absent. Brain MR imaging (3.0-T MR system, Verio, Siemens, AG, Erlangen, Germany) showed bilateral atrophic putamina and head of caudate nuclei on a T2-weighted image (Fig. 1). Peripheral blood smear showed 21% acanthocytes confirmed by scanning electron microscopy (Fig. 2). Finally, genetic testing confirmed a homozygous nonsense mutation in exon 37 (c.4411C>T; p.Arg1471*) in the VPS13 gene. His symptoms did not respond to medications, including diazepam, baclofen, trihexyphenidyl, haloperidol, and tetrabenazine. During the two-year follow-up period, his choreic movement and gait disturbance showed gradual worsening and he was no longer able to walk independently. We recommended DBS for the patient for control of symptoms and for improvement of functional capacity.
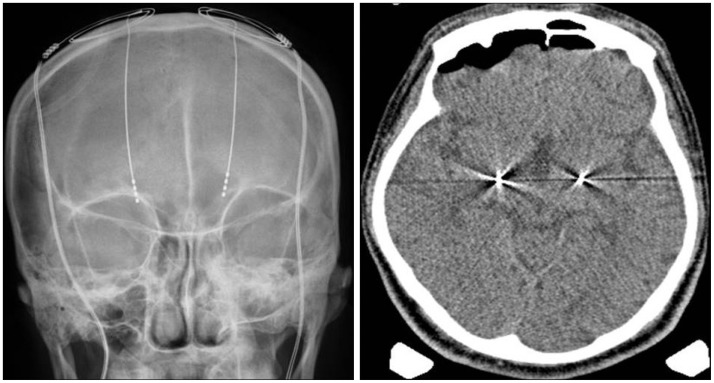
After written consent was obtained, he underwent bilateral implantation of a quadripolar electrode (model 3387; Medtronic, Minneapolis, MN, USA) into the GPi under generalized anesthesia so that the lowest contact terminated at the bottom of GPi (Fig. 3). We performed CT scan in order to confirm adequacy of the electrode locations and absence of hemorrhage followed by placement of an internal pulse generator (Soletra; Medtronic). DBS programming was performed two weeks after implantation. Postoperatively, the benefit was rapidly evident with marked improvement in choreic movements. Tongue and lip biting almost disappeared and he was able to walk independently. However, dysarthria did not show significant improvement. In the six-month postoperative evaluation, motor section of Unified Huntington's Disease Rating Scale (UHDRS) score was 59 in the preoperative evaluation, and 36 after two months. One year after DBS implantation, the score had decreased to 34 and MMSE scores showed no change. The benefit had remained stable for two years (Table 1). The final DBS settings were as follows : right, 2.3 V, 60 µs, 130 Hz; left, 2.9 V, 60 µs, 130 Hz.
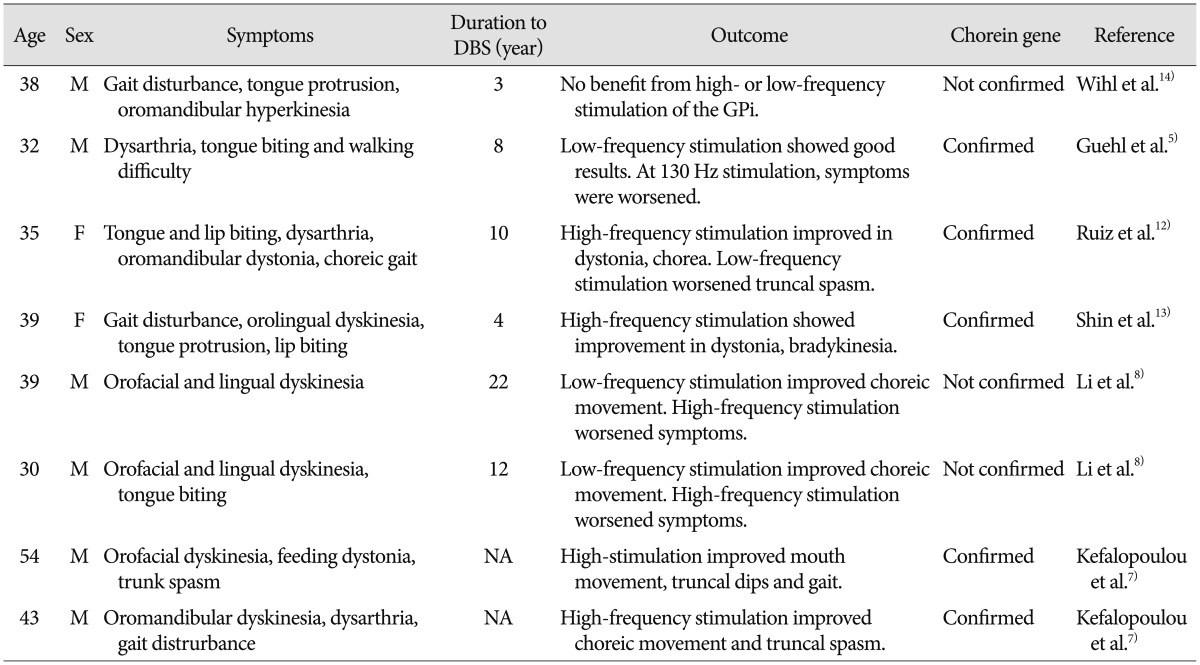
Our patient had typical ChAc, with choreic movements and tongue and lip biting. GPi-DBS provided a rapid benefit, which lasted for two years during the follow-up period. This result supports the usefulness of DBS for symptomatic treatment in cases of ChAc, however, there are still few data regarding long term benefit (Table 2).
DBS has been used in treatment of hyperkinetic movement disorders, including dystonia and L-dopa induced dyskinesia in Parkinson's disease. DBS has recently been employed for relief of symptoms of chorea in patients with Huntington's disease and ChAc. In patients with ChAc, a paucity of case reports of DBS have demonstrated improvements in chorea and activities of daily living, although with some variability in treatment outcome. Only one published case has reported no benefit from DBS14).
One published case of a patient with ChAc reported improvement in choreic movements following DBS of the ventralis oralis posterior nucleus of the thalamus3). However, GPi is a more preferable target for treatment of primary dystonia or neuroacanthocytosis and almost all therapeutic effects were obtained by GPi-DBS. The optimal stimulation parameters remain debatable. High-frequency stimulation has been reported to be effective not only in generalized dystonia, but also in several cases of Huntington's disease1,10), senile chorea15), and cerebral palsy2). In agreement with a few reports7,12,13), our patient showed improvement of his symptoms with high-frequency stimulation (130 Hz). However, the opposite has been reported in some cases of ChAc, where improvement was only achieved with low-frequency stimulation (40 Hz) with worsening at high-frequency stimulation (130 Hz)5,8). The exact reason for these contrasting effects is unclear and conduct of further studies will be needed.
Few studies have reported on the long-term effect of DBS in treatment of ChAc. Miquel et al.9) recently reported a significant long-term improvement of motor symptom severity (improvement ≥20% in UHDRS-motor score) in 61.5% of patients at a mean follow-up period of 2.5 years. They found that gait improvement, orofacial movements and tics, head drops, and trunk spasms were DBS-sensitive symptoms, while dysarthria and feeding problems were less responsive. In our case, during long-term follow-up, the patient showed improvement of 42.3% within one year and 39.0% within two years after surgery. Among his various symptoms, abnormal truncal flexion, tongue and lip biting showed marked improvement within several months after surgery, while dysarthria did not show significant improvement. Resistance to treatment or even worsening of dysarthria has been commonly described in DBS. This has been attributed to the adverse effect of DBS9) or progression of the disease.
ChAc is a rare autosomal recessive disorder characterized by generalized chorea and morphological abnormalities in red blood cells. It presented with orofaciolingual dyskinesia, gait disturbance, and behavioral disturbance. However, the optimal treatment for ChAc is still unclear. Medical treatment is usually ineffective and DBS has been tried in management of ChAc. In recently reported cases, including the one reported here, DBS of the GPi resulted in significant improvement of symptoms in ChAc. Although DBS cannot cure all symptoms of ChAc, it can improve the quality of life for patients. Therefore, DBS is a valuable treatment option and should be considered as a treatment option for ChAc patients who are refractory to medical therapy.
Acknowledgements
The authors would like to thank Chang-Seok Ki, MD, at the Department of Laboratory Medicine and Genetics, Samsung Medical Center, who contributed to genetic confirmation for ChAc. We also appreciate the efforts of Myung-Sik Lee, MD, and Chul-Hyoung Lyoo, MD from the Department of Neurology, Gangnam Severance Hospital, in preparation of the scanning electron microscopic image.
References
1. Bereznai B, Steude U, Seelos K, Bötzel K. Chronic high-frequency globus pallidus internus stimulation in different types of dystonia : a clinical, video, and MRI report of six patients presenting with segmental, cervical, and generalized dystonia. Mov Disord. 2002; 17:138–144. PMID: 11835451.

2. Berweck S. BP-DBS for dystonia-choreoathetosis cerebral palsy. Lancet Neurol. 2009; 8:692–693. PMID: 19576853.

3. Burbaud P, Rougier A, Ferrer X, Guehl D, Cuny E, Arne P, et al. Improvement of severe trunk spasms by bilateral high-frequency stimulation of the motor thalamus in a patient with chorea-acanthocytosis. Mov Disord. 2002; 17:204–247. PMID: 11835468.

4. Dobson-Stone C, Velayos-Baeza A, Filippone LA, Westbury S, Storch A, Erdmann T, et al. Chorein detection for the diagnosis of chorea-acanthocytosis. Ann Neurol. 2004; 56:299–302. PMID: 15293285.

5. Guehl D, Cuny E, Tison F, Benazzouz A, Bardinet E, Sibon Y, et al. Deep brain pallidal stimulation for movement disorders in neuroacanthocytosis. Neurology. 2007; 68:160–161. PMID: 17210902.

6. Hardie RJ, Pullon HW, Harding AE, Owen JS, Pires M, Daniels GL, et al. Neuroacanthocytosis. A clinical, haematological and pathological study of 19 cases. Brain. 1991; 114(Pt 1A):13–49. PMID: 1998879.
7. Kefalopoulou Z, Zrinzo L, Aviles-Olmos I, Bhatia K, Jarman P, Jahanshahi M, et al. Deep brain stimulation as a treatment for chorea-acanthocytosis. J Neurol. 2013; 260:303–305. PMID: 23086180.

8. Li P, Huang R, Song W, Ji J, Burgunder JM, Wang X, et al. Deep brain stimulation of the globus pallidus internal improves symptoms of chorea-acanthocytosis. Neurol Sci. 2012; 33:269–274. PMID: 21863267.

9. Miquel M, Spampinato U, Latxague C, Aviles-Olmos I, Bader B, Bertram K, et al. Short and long term outcome of bilateral pallidal stimulation in chorea-acanthocytosis. PLoS One. 2013; 8:e79241. PMID: 24223913.

10. Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, et al. Bilateral globus pallidus stimulation for Huntington's disease. Ann Neurol. 2004; 56:290–294. PMID: 15293283.

11. Rampoldi L, Dobson-Stone C, Rubio JP, Danek A, Chalmers RM, Wood NW, et al. A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat Genet. 2001; 28:119–120. PMID: 11381253.

12. Ruiz PJ, Ayerbe J, Bader B, Danek A, Sainz MJ, Cabo I, et al. Deep brain stimulation in chorea acanthocytosis. Mov Disord. 2009; 24:1546–1547. PMID: 19425062.

13. Shin H, Ki CS, Cho AR, Lee JI, Ahn JY, Lee JH, et al. Globus pallidus interna deep brain stimulation improves chorea and functional status in a patient with chorea-acanthocytosis. Stereotact Funct Neurosurg. 2012; 90:273–277. PMID: 22777538.

14. Wihl G, Volkmann J, Allert N, Lehrke R, Sturm V, Freund HJ. Deep brain stimulation of the internal pallidum did not improve chorea in a patient with neuro-acanthocytosis. Mov Disord. 2001; 16:572–575. PMID: 11391763.

15. Yianni J, Nandi D, Bradley K, Soper N, Gregory R, Joint C, et al. Senile chorea treated by deep brain stimulation : a clinical, neurophysiological and functional imaging study. Mov Disord. 2004; 19:597–602. PMID: 15133831.

Fig. 2
A : Acanthocytosis (arrows) in a peripheral blood smear (wright stain, ×1000). B : Electron microscopic scanning.
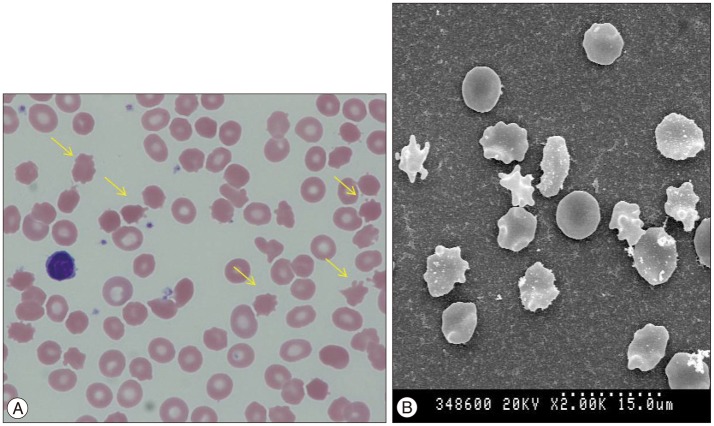
Fig. 3
Plain skull X-ray and CT scan showing a deep brain stimulation electrode located on the globus pallidus bilaterally.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download