Abstract
Neurocutaneous melanosis (NCM) is a rare congenital syndrome consisting of benign or malignant melanotic tumors of the central nervous system with large or numerous cutaneous melanocytic nevi. The Dandy-Walker complex (DWC) is characterized by an enlarged posterior fossa with high insertion of the tentorium, hypoplasia or aplasia of the cerebellar vermis, and cystic dilatation of the fourth ventricle. These each two conditions are rare, but NCM associated with DWC is even more rare. Most patients of NCM with DWC present neurological symptoms early in life such as intracranial hemorrhage, hydrocephalus, and malignant transformation of the melanocytes. We report a 14-year-old male patient who was finally diagnosed as NCM in association with DWC with extensive intracerebral and spinal cord involvement.
Neurocutaneous melanosis (NCM) is a rare dysmorphogenesis associated with single or multiple giant pigmented cutaneous nevi and abnormal proliferation of the melanin-containing cells in the leptomeninges of the central nervous system (CNS). Since the Rokitansky have been described in 1861, fewer than 200 cases of NCM have been reported in literature2,4,8,15,17,21,23,24,26,28). The leptomeninges of the skull base, ventral surface of brain stem and cerebellum are the most affected sites of CNS4,10,11,16,17,25,26). The malignant transformation into malignant melanoma is seen in about 40-64% of cases with death after CNS complications6,10,12,15,17,18,19,25). Di Rocco et al.11) and other authors4,19,20,21) reported that 8% to 10% of patients with NCM have been associated with Dandy-Walker complex (DWC), which was suggested by some authors4,6,10,17,21,23), a new term instead of Dandy-Walker malformation, Dandy-Walker variant, and the mega cistern magna. Spinal involvement may be seen in about 20% of NCM patients10,26).
We would like to report a 14-year-old NCM patient accompanied with DWC who displayed malignant melanoma in the cerebral hemisphere along with extensive malignant infiltration of spinal meninges.
A 14-year-old male patient visited our hospital with headache, nausea, vomiting, dysarthria and right hemiparesis grade I as the main symptoms. Dark brown colored large melanocytic nevus that covered about 1/2 of the trunk and various sized nevus ranging from several millimetre to several centimetre were observed throughout the whole body of the patient (Fig. 1).
When the patient was 6 months old, in the dermatology department of our hospital, histologic examination of nevus sections showed nevus cell infiltration with pigmentation in the reticular dermis. No evidence of malignant transformation was found (Fig. 2). The patient suffered an onset of seizure when he was two years old and received treatment with anticonvulsant medication at the pediatric department of our hospital. Under NCM impression at the time, serial follow-ups for clinical symptoms were done for 12 years by the pediatrics department. During that time, there was no manifestation of abnormal neurological symptoms. The patient visited due to motor weakness. The computed tomography (CT) imaging scans showed intracerebral and intraventricular hemorrhage throughout left posterior frontal cortical area and left lateral ventricle. Under magnetic resonance image (MRI) gadolinum-enhanced T1 weighted image, partially high signal was detected in the cortical area of frontal lobe. Small inhomogenous high signal was also observed on the pons, temporal horn, and anterior portion of lateral ventricle through gadolinum-enhanced T1 weighted image. MRI scans also revealed a cyst in the posterior fossa and hypoplasia of the cerebellar vermis with dilatation of the entire ventricular system. Longitudinal diffuse enhancement lesion was observed along the posterior dura at the thoracic level in the whole spine MRI scans carried out for the purpose of screening but nodular lesion or focal mass lesion was not observed in other areas (Fig. 3). Although surgical treatment under NCM impression accompanies by DWC was recommended, we could not operate on him due to refusal by the guardian. After the conservative care during one month, the patient was discharged with improvement of neurological symptoms. However, the patient was hospitalized again with headache and right hemiparesis grade I after 4 months. Large mass, which is a marked enlarged state of left frontoparietal area, was observed under the MRI scans. The surgical treatment was performed. Tumor along with diffuse dark colored leptomeninges and with soft, friable, vascularized and poorly defined borders of margin was removed gross totally (Fig. 4). Histopathologic examination showed melanin-pigment laden neoplastic cells, necrotic change and pleomorphic malignant cell, which could be diagnosed the malignant melanoma (Fig. 5A-C). No pathologic deposits of the skin area were seen in the positron emission tomography with 18-FDG (Fig. 5D). It was possible to definitively diagnose NCM with DWC. After 2 month following first brain surgery, the patient was discharged with symptom improvement. Three months after discharge, the patient was hospitalized again with observation of increased intracranial pressure symptoms such as decreased mentality, headache and nausea. Re-grown large mass on the left frontoparietal area and new mass on left medial temporal area were observed under MRI scans (Fig. 6). The mass on the left frontoparietal area was removed gross totally once again through surgery with same pathological findings. On the 3rd week following the second surgical treatment, the patient complained of back pain, voiding difficulty, left side motor weakness grade III and respiratory difficulty. So spinal MRI scans was done, and mass lesion with high signal at the T2 weighted image and inhomogenous high signal at gadolinum-enhanced T1 weighted image along the C5-T3 area were observed, and bilateral laminectomy with partial removal of the mass was performed. The intradural mass showed a soft, friable, highly vascularized characteristics and infiltrated into the leptomenignes with extremely poorly defined border (Fig. 7). Although performing aggressive treatment, decreased of consciousness, respiratory difficulty and motor weakness continued in the patient, and progressive hydrocephalus was observed on CT scan. The guardian did not wish further treatment. The patient died on the 3rd week following the spine surgery.
Since the first report was described in 1861, the diagnostic criteria was established by Kadonaga and Frieden in 1991 (Table 1)18). To exclude the cases of cutaneous melanoma with brain metastasis, they suggested the no evidence of cutaneous melanoma except in patients in whom the examined portions of the meningeal lesions are benign. And the cases of definite NCM require histologic confirmation of the CNS lesions. On the other hand, the cases without histologic confirmation of CNS lesions are considered provisional.
NCM is a nonhereditary entity, which occurs equally in both sexes6,12,16,19,23,24,29). The pathogenesis of NCM is not clear. Melanocytes normally exist in human epidermis, hair bulb, leptomeninges, uveal tract and retina26,30), and are thought to be derived from multipotential precursor cells of the neural crest19). And these neural crest cells normally migrate to their final position in embryonic skin by day 50 of development26), and have been detected in fetal epidermis at 8-10 weeks and 5.5 months in the fetal meninges14). In normal development, proper differentiation of these cells into mature melanocytes has been done after the cell migration. Therefore, NCM may be related to a congenital errors in dysmorphogenesis of multipluripotent precursor cells of the embryonal neuroectoderm2,4,7,10,14,19,21,29).
The DWC is a rare developmental disorder of the CNS and their incidence has been estimated to be one per 25000-30000 live births4,19). The DWC is characterized by hypoplasia or aplasia of the cerebellar vermis, cystic dilatation of the posterior fossa, and an enlarged fourth ventricle. Although the pathogenesis of DWC is not clear, it was thought that they are caused by an induction failure of the opposing cerebellar plates and the persistence of the membrane area of the fourth ventricle before the 7th week of embryonic development4,20).
The association of NCM with DWC are very rare and fewer than 30 cases have been reported. We reviewed the 26 literatures2,4,6,7,8,9,10,12,13,14,16,17,19,20,21,22,23,24,25,26,27,28,29,30), and our case. The definite mechanism of the concurrent NCM and DWC is still unknown. The meningeal cells are important in cerebellar development7,9,16,26). And many authors4,6,7,10,11,14,17,19,21,23,24,25,26,28) proposed that excessive melanosis of the leptomeninges may interfere with ectodermal-mesodermal interaction and normal cerebellar development. This developmental interference may lead to the clinical images of the DWC.
When neurologic symptoms occur, NCM generally has a poor prognosis regardless of the presence of malignancy17,19,25,26,29). Furthermore, the concurrent existence of DWC appears to have a more poorer prognosis7,8,11,21,22,26). Several authors6,10,12,15,17,18,19,25) reported that malignant transformation is seen in about 40-64% of cases with death presenting CNS complications. In presence of leptomeningeal malignant melanoma, the patients showed the rapid deterioration and tumor extension to CNS area through the leptomeninges.
The prognosis of provisional NCM (pNCM) with DWC was generally good2,4,6,8,10,13,14,19,25,26,28). Most of their main symptoms were hydrocephalus and seizure. Nine of 11 patients of pNCM with DWC were alive when they were reported. On the other hand, the prognosis of definite NCM (dNCM) with DWC was very poor7,9,12,16,17,20,21,22,23,24,25,27,29,30). The clinical symptoms of dNCM were more severe and the spinal involvement was more frequent9,12,16,23,24). The eleven of 16 patients died of neurologic deterioration. The eight of 11 expired patients presented the malignant melanoma. Among the eight cases, six patients, including our case, showed the malignant transformation to CNS melanoma during the follow-up after diagnosis pNCM9,16,17,22,25). Although the pNCM with DWC has a favorable prognosis, the possibility of the malignant transformation to CNS melanoma has been reported, as mentioned above. And so we suggest that the long-term follow-up will be needed for these pNCM with DWC.
After the diagnosis of NCM with DWC, there is no particular treatment to prevent the malignant change, so the physician needs the closed follow-up for the patients, even if the symptom are stable. In our study, this patient has prolonged survival period of about 14 years since the occurrence of seizure at the age of 2. After the stable period of 12 years, the patient showed the rapid progression of symptom and extension of tumor to brain and spinal cord. We reported a proper case, which presented the clinical progress from the malignant change of NCM to patient's terminal state.
References
1. Akinwunmi J, Sgouros S, Moss C, Grundy R, Green S. Neurocutaneous melanosis with leptomeningeal melanoma. Pediatr Neurosurg. 2001; 35:277–279. PMID: 11741125.

2. Arai M, Nosaka K, Kashihara K, Kaizaki Y. Neurocutaneous melanosis associated with Dandy-Walker malformation and a meningohydroencephalocele. Case report. J Neurosurg. 2004; 100(5 Suppl Pediatrics):501–505. PMID: 15287463.

3. Barkovich AJ, Frieden IJ, Williams ML. MR of neurocutaneous melanosis. AJNR Am J Neuroradiol. 1994; 15:859–867. PMID: 8059652.
4. Berker M, Oruckaptan HH, Oge HK, Benli K. Neurocutaneous melanosis associated with Dandy-Walker malformation. Case report and review of the literature. Pediatr Neurosurg. 2000; 33:270–273. PMID: 11155066.

5. Burstein F, Seier H, Hudgins PA, Zapiach L. Neurocutaneous melanosis. J Craniofac Surg. 2005; 16:874–876. PMID: 16192875.

6. Caceres A, Trejos H. Neurocutaneous melanosis with associated Dandy-Walker complex. Childs Nerv Syst. 2006; 22:67–72. PMID: 15580515.

7. Chaloupka JC, Wolf RJ, Varma PK. Neurocutaneous melanosis with the Dandy-Walker malformation : a possible rare pathoetiologic association. Neuroradiology. 1996; 38:486–489. PMID: 8837100.

8. Cho IY, Hwang SK, Kim SH. Dandy-walker malformation associated with neurocutaneous melanosis. J Korean Neurosurg Soc. 2011; 50:475–477. PMID: 22259699.

9. Craver RD, Golladay SE, Warrier RP, Gates AJ, Nelson JS. Neurocutaneous melanosis with Dandy-Walker malformation complicated by primary spinal leptomeningeal melanoma. J Child Neurol. 1996; 11:410–414. PMID: 8877612.

10. Demirci A, Kawamura Y, Sze G, Duncan C. MR of parenchymal neurocutaneous melanosis. AJNR Am J Neuroradiol. 1995; 16:603–606. PMID: 7793388.
11. Di Rocco F, Sabatino G, Koutzoglou M, Battaglia D, Caldarelli M, Tamburrini G. Neurocutaneous melanosis. Childs Nerv Syst. 2004; 20:23–28. PMID: 14576958.

12. Ellis DS, Spencer WH, Stephenson CM. Congenital neurocutaneous melanosis with metastatic orbital malignant melanoma. Ophthalmology. 1986; 93:1639–1642. PMID: 3808623.

13. Gönül M, Soylu S, Gül U, Aslan E, Unal T, Ergül G. Giant congenital melanocytic naevus associated with Dandy-Walker malformation, lipomatosis and hemihypertrophy of the leg. Clin Exp Dermatol. 2009; 34:e106–e109. PMID: 19438567.

14. Green LJ, Nanda VS, Roth GM, Barr RJ. Neurocutaneous melanosis and Dandy-Walker syndrome in an infant. Int J Dermatol. 1997; 36:356–359. PMID: 9199983.
15. Hsueh CW, Ho CS, Chiu NC, Shen EY. Neurocutaneous melanosis with hydrocephalus : report of one case. Acta Neurol Taiwan. 2004; 13:29–33. PMID: 15315299.
16. Humes RA, Roskamp J, Eisenbrey AB. Melanosis and hydrocephalus. Report of four cases. J Neurosurg. 1984; 61:365–368. PMID: 6737062.
17. Kadonaga JN, Barkovich AJ, Edwards MS, Frieden IJ. Neurocutaneous melanosis in association with the Dandy-Walker complex. Pediatr Dermatol. 1992; 9:37–43. PMID: 1574474.

18. Kadonaga JN, Frieden IJ. Neurocutaneous melanosis : definition and review of the literature. J Am Acad Dermatol. 1991; 24(5 Pt 1):747–755. PMID: 1869648.
19. Kalayci M, Cağavi F, Bayar U, Gül S, Dursun A, Ermis B, et al. Neurocutaneous melanosis associated with Dandy-Walker malformation. Acta Neurochir (Wien). 2006; 148:1103–1106. discussion 1106. PMID: 16489502.

20. Kang SG, Yoo DS, Cho KS, Kim DS, Chang ED, Huh PW, et al. Coexisting intracranial meningeal melanocytoma, dermoid tumor, and Dandy-Walker cyst in a patient with neurocutaneous melanosis. Case report. J Neurosurg. 2006; 104:444–447. PMID: 16572661.

21. Kim KH, Chung SB, Kong DS, Seol HJ, Shin HJ. Neurocutaneous melanosis associated with Dandy-Walker complex and an intracranial cavernous angioma. Childs Nerv Syst. 2012; 28:309–314. PMID: 22134415.

22. Livingstone E, Claviez A, Spengler D, Barth H, Stark AM, Hugo HH, et al. Neurocutaneous melanosis : a fatal disease in early childhood. J Clin Oncol. 2009; 27:2290–2291. PMID: 19349541.
23. Marnet D, Vinchon M, Mostofi K, Catteau B, Kerdraon O, Dhellemmes P. Neurocutaneous melanosis and the Dandy-Walker complex : an uncommon but not so insignificant association. Childs Nerv Syst. 2009; 25:1533–1539. PMID: 19711088.

24. McClelland S 3rd, Charnas LR, SantaCruz KS, Garner HP, Lam CH. Progressive brainstem compression in an infant with neurocutaneous melanosis and Dandy-Walker complex following ventriculoperitoneal shunt placement for hydrocephalus. Case report. J Neurosurg. 2007; 107(6 Suppl):500–503. PMID: 18154021.

25. Mena-Cedillos CA, Valencia-Herrera AM, Arroyo-Pineda AI, Salgado-Jiménez MA, Espinoza-Montero R, Martínez-Avalos AB, et al. Neurocutaneous melanosis in association with the Dandy-Walker complex, complicated by melanoma : report of a case and literature review. Pediatr Dermatol. 2002; 19:237–242. PMID: 12047644.
26. Pavlidou E, Hagel C, Papavasilliou A, Giouroukos S, Panteliadis C. Neurocutaneous melanosis : report of three cases and up-to-date review. J Child Neurol. 2008; 23:1382–1391. PMID: 19073843.

27. Reyes-Mugica M, Chou P, Byrd S, Ray V, Castelli M, Gattuso P, et al. Nevomelanocytic proliferations in the central nervous system of children. Cancer. 1993; 72:2277–2285. PMID: 8374887.

28. Schreml S, Gruendobler B, Schreml J, Bayer M, Ladoyanni E, Prantl L, et al. Neurocutaneous melanosis in association with Dandy-Walker malformation: case report and literature review. Clin Exp Dermatol. 2008; 33:611–614. PMID: 18477004.

29. Walbert T, Sloan AE, Cohen ML, Koubeissi MZ. Symptomatic neurocutaneous melanosis and Dandy-Walker malformation in an adult. J Clin Oncol. 2009; 27:2886–2887. PMID: 19398571.

30. Yu HS, Tsaur KC, Chien CH, Perng JJ, Lu CC. Neurocutaneous melanosis : electron microscopic comparison of the pigmented melanocytic nevi of skin and meningeal melanosis. J Dermatol. 1985; 12:267–276. PMID: 3908531.
Fig. 2
The skin showed nevus cell infiltration with pigmentation in the reticular dermis (hematoxylin and eosin, ×40).
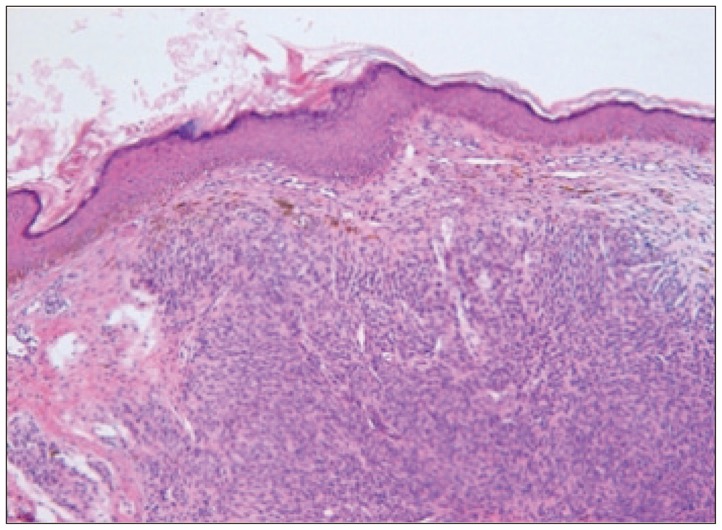
Fig. 3
T1-weighted MR scan images of brain and spine with gadolinium injection performed show significant enhancement of cortex in posterior frontal area (brain axial), significant enhancement of pons and hypoplasia of superior or inferior cerebellar vermis and both cerebellar hemisphere with dilatation of the inferior fourth ventricle, cystic enlargement of the posterior fossa (brain sagittal), and extensive enhancement of leptomeninges in spinal cord (spine sagittal).
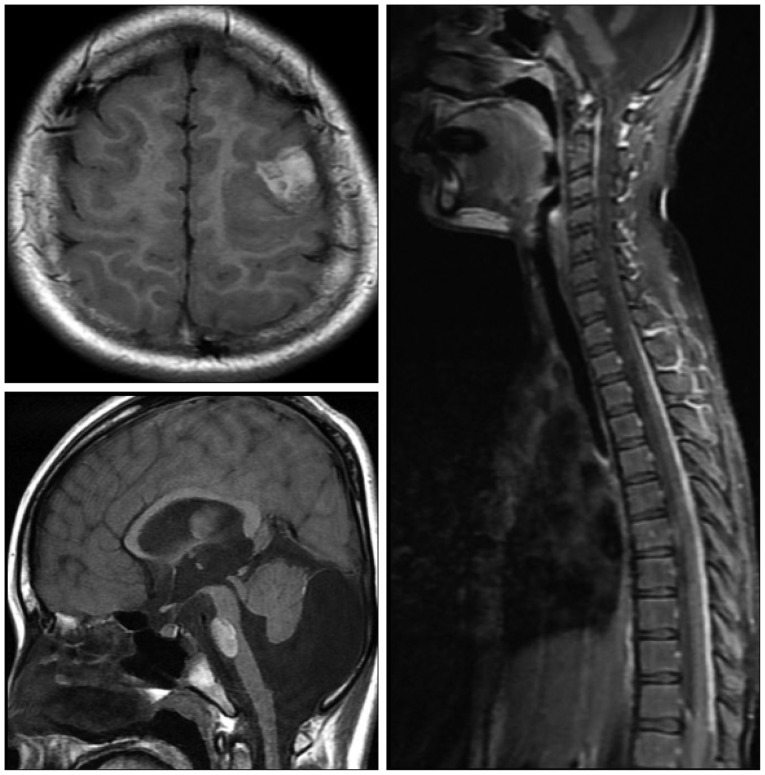
Fig. 4
T1-weighted MR scan (axial and sagittal) images with gadolinium injection performed show significant enhancement of large mass lesion in left frontoparietal area. Intraoperative view shows black diffuse infiltration of leptomeninges and large mass.
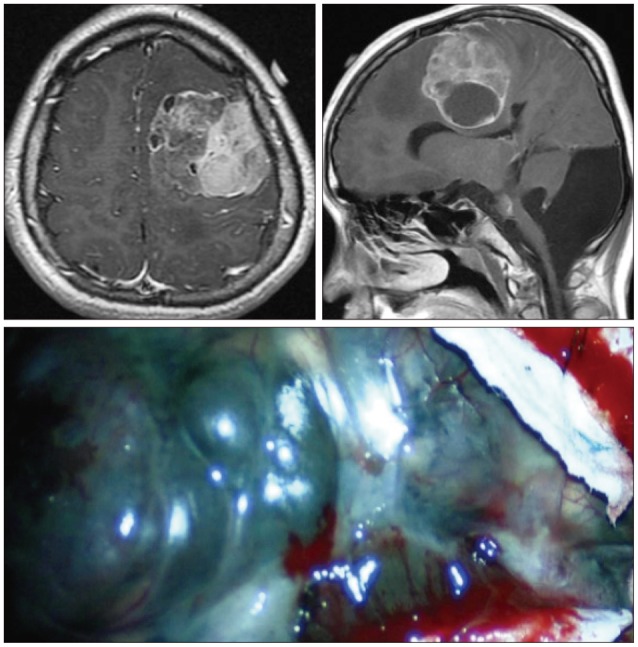
Fig. 5
Pathological findings show diffuse or solid pattern of small to medium sized malignant cells with dense and hyperchromatic round nuclei and scanty cytoplasm [hematoxylin and eosin (H&E), ×400] (A). Extensive geographic shaped necrosis surrounded by pallisading malignant cells also noted (H&E, ×600) (B). Melanin-pigment laden neoplastic cells are frequently observed (Melanin A, ×400) (C). The positron emission tomography with 18-FDG shows no pathologic deposits of the skin area (D).
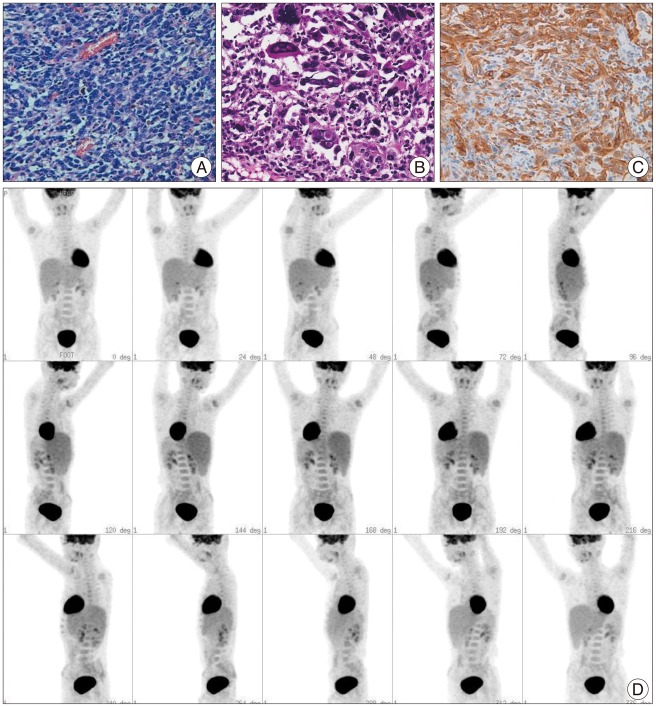
Fig. 6
T1-weighted MR scan images with gadolinium injection performed. Significant enhancement of recurred mass lesion in left frontoparietal area (A) and new mass on left medial temporal area are observed (B).
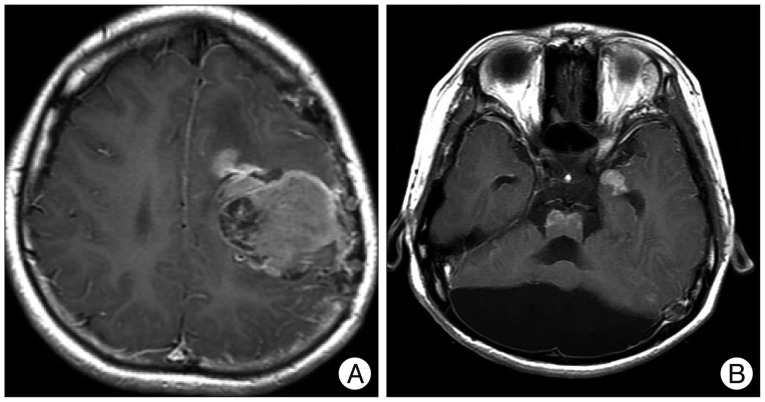
Fig. 7
T1-weighted MR scan images of spine performed after gadolinium injection show extensive enhancement of leptomeninges involving whole spinal cord and new mass lesion in lower cervix and upper thorax. Intraoperative view in spine shows black diffuse infiltration of leptomeninges with extremely poorly defined border and mass.
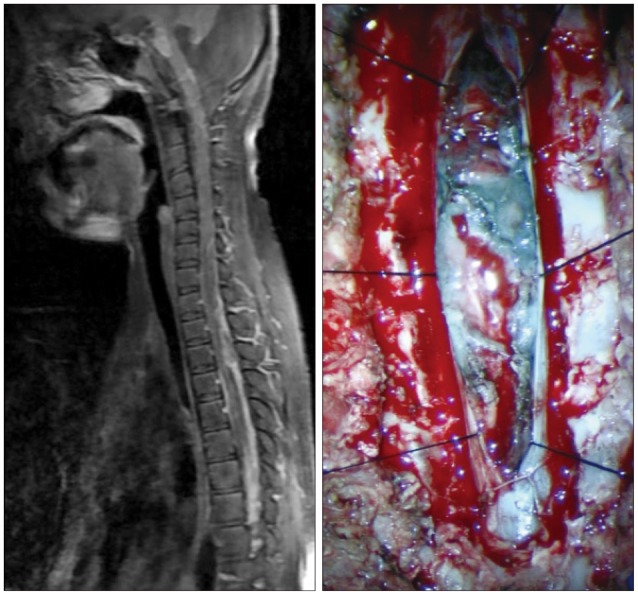
Table 1
NCM criteria of Kadonaga and Frieden
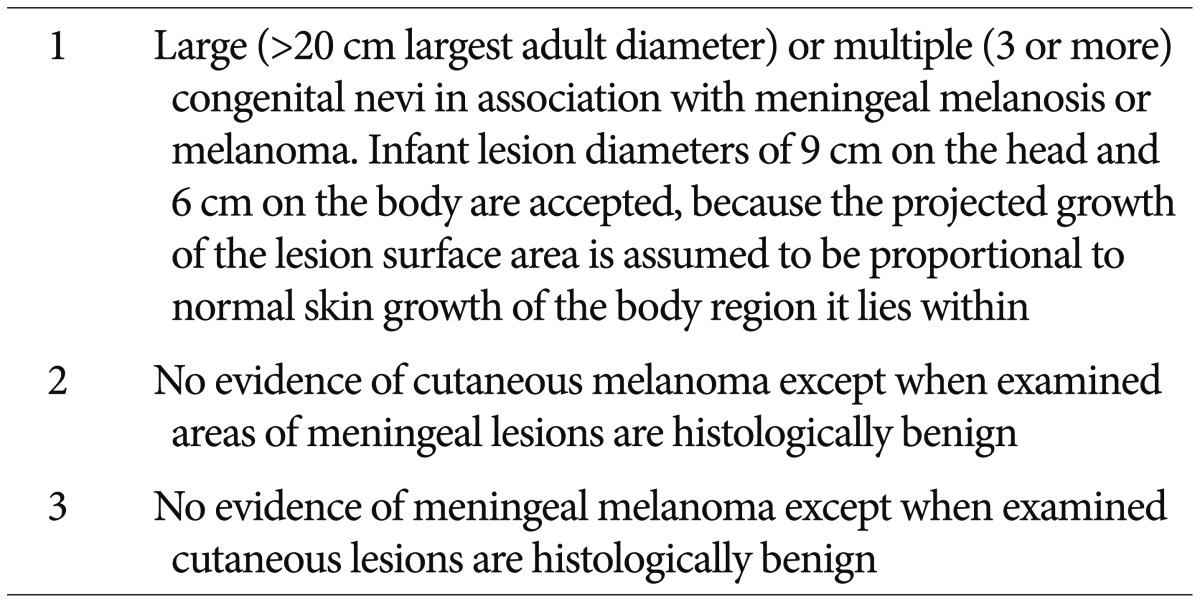
Adopted from Kadonaga JN, Frieden IJ : J Am Acad Dermatol 24 (5 Pt 1) : 747-755, 199118). NCM : neurocutaneous melanosis




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download