Abstract
Sympathetic dysfunction is one of the possible complications of anterior spine surgery; however, it has been underestimated as a cause of complications. We report two successful experiences of treating severe dysesthetic pain occurring after anterior spine surgery, by performing a sympathetic block. The first patient experienced a burning and stabbing pain in the contralateral upper extremity of approach side used in anterior cervical discectomy and fusion, and underwent a stellate ganglion block with a significant relief of his pain. The second patient complained of a cold sensation and severe unexpected pain in the lower extremity of the contralateral side after anterior lumbar interbody fusion and was treated with lumbar sympathetic block. We aimed to describe sympathetically maintained pain as one of the important causes of early postoperative pain and the treatment option chosen for these cases in detail.
Anterior cervical and lumbar spine surgery is one of the most commonly performed spinal procedures. Anterior cervical discectomy and fusion (ACDF) is an established surgical procedure for the management of patients with cervical radiculopathy and myelopathy secondary to degenerative disc disease and spondylosis2). Various complications related to anterior cervical spine surgery including postoperative recurrent laryngeal nerve palsy, Horner syndrome, pharyngeal/esophageal laceration and vessel injury have been reported8). Anterior lumbar surgery is also commonly used for the treatment of degenerative disc disease, spondylolisthesis, tumor, infection and fracture. This approach has many advantages including excellent exposure of the operation field, a thorough discectomy can be performed, and no disruption of the paravertebral muscle and ligament. Anterior lumbar surgery-related complications reported in the literature have been mainly focused on vascular injury, infection and gastrointestinal issues4,21).
Sympathetic dysfunction is one of the possible complications of anterior spine surgery; however, it has been underestimated. It manifests in various forms. In the current report, our two patients presented with dysesthetic pain in the upper and lower extremities soon after cervical and lumbar surgery using an anterior approach, respectively. Their pain was dramatically relieved after a block of the stellate ganglion and lumbar sympathetic ganglion, respectively.
Only few literatures describe the rare occurrence of sympathetic dysfunction related pain developing after anterior spine surgery and the treatment13,22). We aimed to report two successful experiences of treating dysesthetic pain occurring immediately after anterior cervical and lumbar surgery by using a sympathetic block and to describe the sympathetically maintained pain (SMP) as one of the important causes of early postoperative pain.
A 43-year-old man with a 3-month history of burning dysesthetic pain and tightness in both upper limbs but mainly in the right upper extremity, which developed after three cervical spine operations, was referred to our hospital with opioid, gabapentin and amitriptyline medications. Five months prior to admission, he underwent 6-mm artificial disc replacement at the C6/7 level through a left anterior approach because of radiculopathy of the right C7 dermatome due to a herniated intervertebral disc at the right paracentral and foraminal region of C6/7 (Fig. 1). After the surgery his pain was reduced, however, similar pain recurred a month after the operation. Two months after the first operation, he underwent C5/6 anterior cervical discectomy through a left ACDF after a diagnostic right C5/6 transforaminal epidural block, with relief of symptoms. On the day following the second operation, he started to experience an unexpected burning and stabbing pain that he rated as 9/10 on a numeric rating scale (NRS) in both upper extremities below the elbow, especially in the right hand. Plain radiographs and MRI of the cervical spine obtained immediately after the procedure showed no evidence of soft tissue hematoma or mechanical failure of the implanted instrumentation (Fig. 2). Finally, ten days after the second operation, he underwent exploration and replacement of the 6-mm height artificial disc with a 5.25-mm height disc at C6/7 due to a suspicion of a possible stretching injury of the cervical spinal nerve. Unfortunately, even after the surgery, his severe pain and tightness persisted. He has begun to take opioid medication and underwent a bilateral C5/6 and C6/7 transforaminal epidural block, right suprascapular nerve block and trigger point injections, but with no effect.
At initial evaluation in our hospital, he presented with chest discomfort, mild hoarseness, tightness and insomnia, neck pain and severe dysesthetic pain in a non-dermatomal pattern in the right upper extremities, at the level of 9/10, by NRS. Physical examination revealed intact motor strength. Laryngoscopic examination by an otolaryngologist revealed slightly decreased mobility of vocal cords on the left side, which indicated a clinical diagnosis of injury of the left recurrent laryngeal nerve.
We performed a right stellate ganglion block (SGB) with 5 mL of 0.5% mepivacaine. After the procedure, his pain and tightness of the right arm reduced to 4/10 on the NRS. He underwent serial six right SGBs for two weeks, with gabapentin and amitriptyline medications. After this, his pain reduced dramatically to 0/10 on the NRS. During the one-year following the treatment, the patient reported three intermittent episodes of mild right arm pain, but overall the pain has been satisfactorily controlled with SGB.
A 69-year-old woman with a 2-month history of right lateral calf and foot pain was referred to our hospital. She had a history of undergoing anterior lumbar interbody fusion (ALIF) through a left retroperitoneal approach, decompressive laminectomy and posterior fusion from L3 to S1 due to pain in the lower back and both lower extremities two months before presenting to our hospital. The preoperative pain was especially severe on the left due to a herniated intervertebral disc at the left foraminal region of L5/S1 and degenerative spondylolisthesis at the L3/4 and L4/5 levels (Fig. 3). These operations had relieved the pain in left lower extremity. However, she began experiencing a severe unexpected pain 9/10 on the NRS in the right lower extremity below the knee and complained of a cold sensation of the right foot without any change of great toe dorsiflexion following the last operation. She had begun to take opioid medication. The right L3/4, L4/5, and L5/S1 transforaminal epidural block did not reduce her pain significantly. A doppler sonography of both lower extremities that was done to rule out deep vein thrombus was normal. Two weeks after the first operation, she received right L4/5 and L5/S1 facetectomy and decompression surgery from the right L4 to S1 nerve root. Her pain and dysethesia of the right calf and foot at the level of 9/10 on the NRS persisted. She underwent subsequent right sacroiliac joint injection, right L4/5, L5/S1 transforaminal epidural block and percutaneous epidural adhesiolysis, especially focused on the right L5/S1 foramen (Fig. 4A), but this also had no effect. Finally, she underwent additional decompression surgery at the right L5/S1 extraforaminal region (Fig. 4B). Unfortunately, the pain was not relieved, and the patient was referred to our hospital. At this time, the pain was not controlled despite being on a pain management regimen consisting of opioid, gabapentin and amitriptyline medications.
On arrival at our hospital, she complained of dysesthetic pain in the right lower extremity below the knee, especially her foot, in a non-dermatomal pattern of an intensity level of 9/10 on the NRS. Physical examination revealed intact motor strength. We decided to perform right lumbar sympathetic block (LSB) at L3 with 10 mL of 0.36% ropivacaine (Fig. 5). The temperature of the ipsilateral sole increased more than 2℃ after the procedure. She reported reduced pain from 9/10 to 4/10 on the NRS for 5 hours following the procedure. Three days after the LSB, we performed radiofrequency lesioning of the right lumbar sympathetic chain at the L2 and L3 levels. Her pain gradually subsided and she reported prolonged warmth in her right foot. During the six-months following the treatment, she reported no recurrence of her pain without opioid, gabapentin and amitriptyline medications.
The chronic postoperative pain is located in an axial and radicular distribution and may be referred to other regions. The character of the pain can be presented in a somatic or neuropathic pattern. Poorly defined and vaguely located pain may be present in a nonphysiologic distribution, and Onesti20) stated that this sort of pain could be associated with medicolegal and psychosocial issues.
Our two patients complained of severe dysesthetic pain in the early postoperative periods. In this period, inadequate pain relief by surgery at the wrong level and incomplete decompression, postoperative instability, nerve root irritation by surgical hardware, and damage to the muscle and nerve plexus by improper positioning of the patient during prolonged surgery can be sources of pain20,23). However, these causes usually result in somatic pain in a radicular distribution. On the other hand, our patients presented with pain that had neuropathic features in its nonphysiologic distribution; the pain occurred in both upper extremities, mainly in the contralateral side of anterior spinal approach, in a patient who had undergone cervical surgery, and below the knee of the contralateral lower extremity in a patient who had undergone the anterior lumbar surgery. Furthermore, before referral to our hospital, both patients had already undergone precise interventional pain procedures at the corresponding level as seen in the MRI and CT, and additional decompression surgery with no effect. Therefore, we concluded that the pain of both patients was attributable to sympathetic dysfunction related to surgery.
The cervical sympathetic chain including the stellate ganglion is located on the anterior surface of the longus colli muscle, which is an important landmark on the anterior cervical spine and extends from the anterior tubercle of the atlas to the third thoracic vertebral body1,7). For anterior cervical operations, once the prevertebral fascia is opened and the correct level is confirmed, the longus colli muscles are explored on the contralateral anterior side of the spinal lesion. Dissection continues out to the uncinate processes and self-retaining retractors are placed beneath the medial edges of the reflected longus colli muscles12). Because of its anatomic location, the cervical sympathetic trunk is at potential risk of injury during the anterior approach to the cervical vertebral bodies5). Surgery on our patients was performed via the left anterior approach. We regarded the pain to be due to injury of the sympathetic chain of the left side and the resultant relative sympathetic hyperactivity of the right side that had begun since the second operation.
The lumbar sympathetic chain lies in extreme close proximity to the anterolateral aspect of the vertebral bodies and disc spaces between T12 and L4. Therefore, it is also extremely vulnerable to injury during anterolateral exposure of the vertebral bodies and some previous articles have noted that sympathetic nerve injury is unavoidable during surgery with an anterior approach23,25). We considered our second patient also experienced her pain due to injury of the sympathetic chain of the left side and the resultant relative sympathetic hyperactivity of the right side since the first operation.
Sympathetic dysfunction causes temperature variation, paresthesia, dysesthetic pain, discoloration, swelling of the corresponding extremity (upper versus lower extremities), gastrointestinal problems and ejaculatory disturbances in the case of lumbar sympathetic dysfunction22). It has been reported that most patients have no long-term sequelae, and a minority suffer from prolonged dysesthetic pain16,22). But prolonged pain can generate repetitive impulses to the spinal cord and the pain can progress to chronic neuropathic pain. That is an important reason to detect the possible causes of the pain and provide early treatment. The patient described in case 2, besides the pain, presented with ileus for postoperative 5 days. Ileus is often self-limited and usually related to major intraoperative fluid shifts, narcotic use and extensive dissection as well as sympathetic dysfunction. Patients most commonly complain that their contralateral extremity is cold as a result of unilateral lumbar sympathetic nerve injury. This sensation is a result of patients feeling warmth in the ipsilateral extremity due to unopposed vasodilation by parasympathetic fibers3). Our second patient complained of a cold sensation and dysesthetic pain in the right lower extremity below the knee since she undergoing ALIF via the left retroperitoneal approach. It is thought that because the surgery resulted in damage to the sympathetic chain of the left side, there was relative sympathetic hyperactivity of the right side.
Sympathetic overactivity is found in a SMP. Sympathetic nerve block is a common practice for reducing sympathetic tone18). Blockade of the sympathetic nervous system is traditionally accomplished at the stellate ganglion or lumbar sympathetic chain, depending on the location of the pain (upper versus lower extremity)10). SGB is used to treat SMP, pain from vascular insufficiency of the upper extremities and orofacial region by decreasing sympathetic activity14,19). LSB is a widely used modality for the evaluation and management of SMP in the lower extremities including circulatory insufficiency, a variety of peripheral neuropathy and cancer-related complications24,25,26). The complications of LSB include genitofemoral neuralgia, necrosis of the psoas muscle, injury of the kidney and ureter, bleeding, hypotension and impotence11). However, serious complications related to LSB are rare and the procedure is commonly performed in the outpatient unit. If LSB gives effective results, RF lesioning of the lumbar sympathetic chain can be performed in carefully selected patients15).
The precise mechanism that only a small number of patients would suffer from SMP although sympathetic chain injuries are common during anterior spine surgery remains unclear. In fact, it is not completely understood why certain individuals develop SMP in many neuropathic conditions including post-herpetic neuralgia, trigeminal neuralgia, diabetic neuropathy and cancer pain. It has been postulated that it occurs due to neuronal ischemic changes or an autoimmune phenomenon involving ß-adrenergic receptors6). In our patients, pre-existing increased sympathetic tone before anterior spinal surgery is also considered to lead to manifest dysesthetic pain. In clinical practice, increased sympathetic tone is often observed in patients with chronic spinal pain. There is a complex relationship between the somatosensory and sympathetic nervous systems, and chronic pain has been well established to induce changes in the sympathetic nervous system6). Besides that, the origin of the sympathetic chain is the thoracolumbar (T1-L3) spinal cord, in contrast to the craniosacral distribution of the parasympathetic nervous system17). Therefore, the lumbar spinal stenosis, which usually exist below L2/3, compress cauda fibers containing parasympathetic fibers while sympathetic fibers already left the spinal canal above the level of stenosis9). This can explain the existence of increased lumbar sympathetic tone in the lumbar stenosis. For the first patient, it is thought that the sympathetic tone of the right side was already elevated before surgery, and ACDF through a left anterior approach caused the injury to the sympathetic chain of the left side, leaving behind the increased sympathetic tone of the right side. Similarly, for the second patient, it is postulated that spondylolisthesis and lumbar spinal stenosis induced increased the bilateral sympathetic tone before surgery although the symptom was especially severe on the left, and ALIF with a left approach resulted in the injury to the sympathetic chain of the left side only. Further study is needed to conclude the mechanism of developing SMP.
Prolonged pain without early intervention can result in intractable neuropathic pain. Early postoperative pain should not be dismissed or overlooked, and it is important to seek possible causes and provide proper interventional pain management. When patients present with early postoperative pain of a neuropathic nature and other possible causes have been ruled out, sympathetic dysfunction should be considered in the differential diagnosis of the severe dysesthetic pain. A sympathetic block, the stellate ganglion for the upper extremities and the lumbar sympathetic chain for the lower extremities, is an effective method for relieving pain due to sympathetic dysfunction.
References
1. Ateş Y, Asik I, Ozgencil E, Açar HI, Yağmurlu B, Tekdemir I. Evaluation of the longus colli muscle in relation to stellate ganglion block. Reg Anesth Pain Med. 2009; 34:219–223. PMID: 19436184.

2. Barnes B, Haid RW, Rodts GE, Subach BR, Kaiser M. Early results using the Atlantis anterior cervical plate system. Neurosurg Focus. 2002; 12:E13. PMID: 16212326.

3. Bell GR. Complications of lumbar spine surgery. In : Wiesel SW, editor. The Lumbar Spine. ed 2. Philadelphia, PA: Saunders;1996. p. 945–969.
4. Cho CB, Ryu KS, Park CK. Anterior lumbar interbody fusion with stand-alone interbody cage in treatment of lumbar intervertebral foraminal stenosis : comparative study of two different types of cages. J Korean Neurosurg Soc. 2010; 47:352–357. PMID: 20539794.

5. Civelek E, Karasu A, Cansever T, Hepgul K, Kiris T, Sabanci A, et al. Surgical anatomy of the cervical sympathetic trunk during anterolateral approach to cervical spine. Eur Spine J. 2008; 17:991–995. PMID: 18548289.

6. Crockett A, Panickar A. Role of the sympathetic nervous system in pain. Anaesth Intensiv Care Med. 2011; 12:50–54.

7. Ebraheim NA, Lu J, Yang H, Heck BE, Yeasting RA. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine (Phila Pa 1976). 2000; 25:1603–1606. PMID: 10870134.

8. Fielding JW. Complications of anterior cervical disk removal and fusion. Clin Orthop Relat Res. 1992; (284):10–13. PMID: 1395278.

9. Gempt J, Rothoerl RD, Grams A, Meyer B, Ringel F. Effect of lumbar spinal stenosis and surgical decompression on erectile function. Spine (Phila Pa 1976). 2010; 35:E1172–E1177. PMID: 20881655.

10. Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, et al. Complex regional pain syndrome : practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013; 14:180–229. PMID: 23331950.

11. Hong JH, Oh MJ. Comparison of multilevel with single level injection during lumbar sympathetic ganglion block : efficacy of sympatholysis and incidence of psoas muscle injection. Korean J Pain. 2010; 23:131–136. PMID: 20556215.

12. Kavanagh RG, Butler JS, O'Byrne JM, Poynton AR. Operative techniques for cervical radiculopathy and myelopathy. Adv Orthop. 2012; 2012:794087. PMID: 21991427.

13. Knoeller SM, Ehmer M, Kleinmann B, Wolter T. CRPS I following artificial disc surgery : case report and review of the literature. Eur Spine J. 2011; 20(Suppl 2):S278–S283. PMID: 21274730.
14. Lynch ME, Elgeneidy AK. The role of sympathetic activity in neuropathic orofacial pain. J Orofac Pain. 1996; 10:297–305. PMID: 9161234.
15. Manjunath PS, Jayalakshmi TS, Dureja GP, Prevost AT. Management of lower limb complex regional pain syndrome type 1 : an evaluation of percutaneous radiofrequency thermal lumbar sympathectomy versus phenol lumbar sympathetic neurolysis--a pilot study. Anesth Analg. 2008; 106:647–649. PMID: 18227328.

17. Morgan GE, Mikhail MS, Murray MJ. Clinical Anesthesiology. ed 4. New York: Lange Medical Books/McGraw Hill Medical Pub. Division;2006. p. 243.
18. Nelson DV, Stacey BR. Interventional therapies in the management of complex regional pain syndrome. Clin J Pain. 2006; 22:438–442. PMID: 16772798.

19. Noma N, Kamo H, Nakaya Y, Dezawa K, Young A, Khan J, et al. Stellate ganglion block as an early intervention in sympathetically maintained headache and orofacial pain caused by temporal arteritis. Pain Med. 2013; 14:392–397. PMID: 23332006.

21. Quraishi NA, Konig M, Booker SJ, Shafafy M, Boszczyk BM, Grevitt MP, et al. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur Spine J. 2013; 22(Suppl 1):S16–S20. PMID: 23250515.

22. Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999; 91(1 Suppl):60–64. PMID: 10419370.

23. Samudrala S, Khoo LT, Rhim SC, Fessler RG. Complications during anterior surgery of the lumbar spine : an anatomically based study and review. Neurosurg Focus. 1999; 7:e9. PMID: 16918208.
24. Tran KM, Frank SM, Raja SN, El-Rahmany HK, Kim LJ, Vu B. Lumbar sympathetic block for sympathetically maintained pain : changes in cutaneous temperatures and pain perception. Anesth Analg. 2000; 90:1396–1401. PMID: 10825327.

25. Watkins R. Anterior lumbar interbody fusion surgical complications. Clin Orthop Relat Res. 1992; (284):47–53. PMID: 1395313.

26. Woo JH, Park HS, Kim SC, Kim YH. The effect of lumbar sympathetic ganglion block on gynecologic cancer-related lymphedema. Pain Physician. 2013; 16:345–352. PMID: 23877450.
Fig. 1
Preoperative T2 weighted cervical MRI shows herniated intervertebral disc at right paracentral and foraminal region of C6/7 (arrow).

Fig. 2
Postoperative anteroposterior (A), lateral (B) images reveal artificial disc replacement, C5/6 anterior cervical discectomy and fusion state and no evidence of mechanical failure of implanted instrumentation. Postoperative MRI (C) obtained after three operations shows good neural decompression and no evidence of unusual postoperative findings.
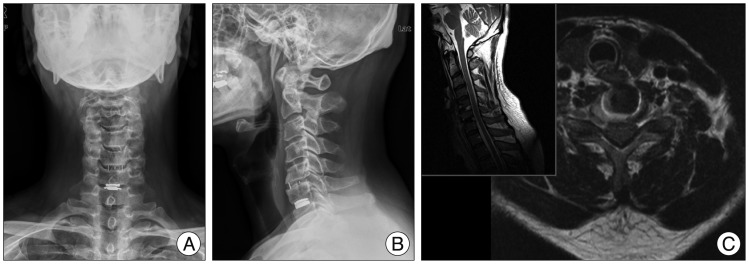
Fig. 3
Preoperative anteroposterior (A) and lateral (B) images of the lumbar spine reveal L3/4 and L4/5 spondylolisthesis. Preoperative T2 weighted lumbar MRI of L5/S1 level shows upward-migrated herniated intervertebral disc (arrow) at left foraminal region (C) and bilateral foraminal stenosis (D). Anteroposterior (E), lateral (F) images obtained after anterior lumbar interbody fusion, decompressive laminectomy and posterior fusion from L3 to S1.
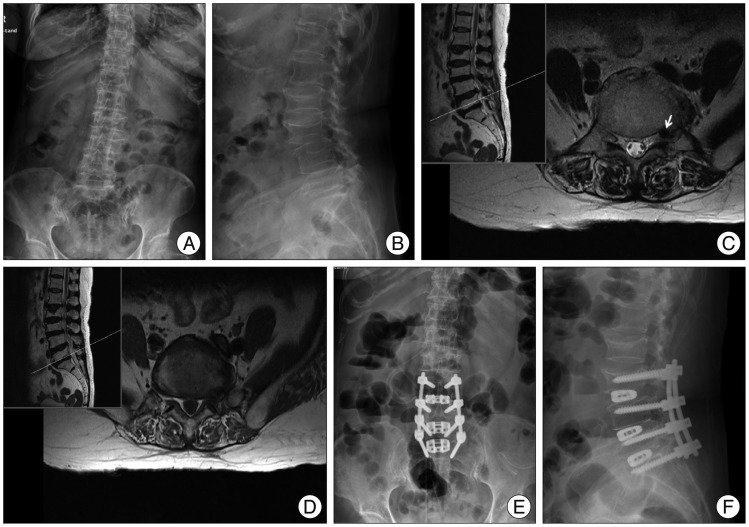
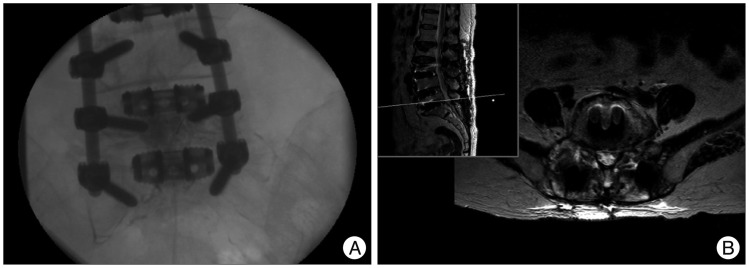
Fig. 4
Images obtained during percutaneous epidural neuroplasty (A). It was performed especially focused on right L5/S1 foramen. MRI obtained after the third operation (B), showing the same level as Fig. 3C, revealed facetectomy and decompression state at right L5/S1 extraforaminal region.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download