Abstract
Primary intraosseous meningioma is a rare tumor, and atypical pathologic components both osteolytic lesion and dura and soft tissue invasion is extremely rare. A 65-year-old woman presented with a 5-month history of a soft mass on the right frontal area. MR imaging revealed a 4 cm sized, multilobulated, strongly-enhancing lesion on the right frontal bone, and CT showed a destructive skull lesion. The mass was adhered tightly to the scalp and dura mater, and it extended to some part of the outer and inner dural layers without brain invasion. The extradural mass and soft tissue mass were totally removed simultaneously and we reconstructed the calvarial defect with artificial bone material. The pathological study revealed an atypical meningioma as World Health Organization grade II. Six months after the operation, brain MR imaging showed that not found recurrence in both cranial and spinal lesion. Here, we report a case of primary osteolytic intraosseous atypical meningioma with soft tissue and dural invasion.
"Primary intraosseous meningioma (PIOM)" is a term used to describe a subset of extradural meningiomas that arise in bone, and it is a subtype of primary extradural meningioma (PEM) that accounts for 1 to 2% of all meningiomas16). It represents approximately two-thirds of all extradural meningiomas7). Especially, among all PIOMs, PIOMs with both osteolytic radiological features and atypical pathological features are extremely rare3,11,12,15,20). In addition, there are few reports about dural involvement of the PIOM3,12). Such PIOMs are usually mistaken for primary bone tumors and appear more prone to develop malignant features than do intracranial meningiomas6). In this report, we describe a case of PIOM of the frontal cranial bone with atypical pathology, which did not metastasize to the whole spine but infiltrated the dural and soft tissues and review the literature of them.
A 65-year-old woman presented with a 5 months history of a soft, enlarging mass in the right frontal region. The scalp mass was painless and there was no history of tauma. On admission, her neurological examination was within the normal range. Skull X-rays and a computed tomography (CT) of the head clearly showed a bony, destructive lesion involving the scalp of the right frontal bone (Fig. 1A). Magnetic resonance (MR) imaging revealed a 6×6 cm heterogeneously enhancing, bony expansive mass in the right frontal bone, extending into the intracranial space with little brain edema (Fig. 1B, C, D). Preoperative diagnosis was a primary or secondary malignant osteolytic mass. We checked the lesions in the whole body of patients using a bone scan, and we performed whole body positron emission tomography CT to rule out metastatic lesions, but abnormal lesions were not found. We performed a craniotomy for excision of the mass. The scalp could be reflected easily and the mass was not adhered to it tightly, but it had partially infiltrated the subcutaneous tissue. The inner and outer table of the skull was destroyed by the well-encapsulated, soft mass. The main tumor appeared to arise extradurally, but definite invasion of the outer and inner dura was observed without brain invasion; therefore we had to remove whole layer of the infiltrated dura including some of the normal dura and we repaired the dura using an artificial component. The mass involving the scalp, skull, and dura was totallyremoved grossly. The calvarial defect was reconstructed with polymethyl methacrylate. The biopsy confirmed the tumor as an atypical (World Health Organization grade II) meningioma (Fig. 2), which caused soft tissue and dural invasion. Pathologic findings showed immunopositive for epithelial membrane antigen, frequent mitosis (Fig. 2A) and necrosis with 10% of Ki-67 labeling index (Fig. 2B). Her post-operative recovery was uneventful and she was discharged on the 14th day post-surgery. The adjuvant treatment was not performed because the mass was totally removed and there were no metastatic lesions. The patients was doing well without any symptoms, and six months postoperatively, we performed follow-up brain MR imaging for assessing if there were any recurrent lesions, and no recurrent mass was observed in the skull, soft tissue, and intracranial area (Fig. 3).
To date, there are a few reports on primary osteolytic intraosseous meningioma, but tumors with atypical features and simultaneous dural involvement have been reported in only 6 cases including the present case (Table 1). Intraosseous meningiomas are rare tumor that originate in the skull and represent the most common type of extradural meningiomas, which accounts for 1-2% of all meningiomas7). The term "primary extradural meningioma (PEM)" indicates that this tumor originates outside the dural layer of any part of the brain or spinal cord and does not have any connection to the dura mater or any intracranial structures12). Hoye et al.9) also emphasized that ectopic meningiomas do not have any connection with the foramina of any cranial nerves or with any intracranial structures. On the other hand, other reports demonstrated that PEM can show intracranial growth involving the dura mater. Bassiouni et al.3) suggested that 14 of 16 (88%) PEM patients who underwent surgery had a true dural involvement, which was proven pathologically. In another report, the inner and outer dura seemed to be uninvolved by the tumor in the intraoperative finding, but a tumor infiltration to inner and outer dura was pathologically proven19). Therefore, "dural involvement" of the PIOM is an issues that can cause confusinon regarding the definition of the PEM and the exact definition has not been determined.
Many different hypotheses exist regarding the origin of primary extradura meningiomas. The development of extradural meningiomas has been explained by entrapment of arachnoid cap cell along the cranial sutures during molding of the head at birth2). It has been also assumed that meningioma may originate from dura and arachnoid that was captured at suture line or fracture sites after traumatic insults4). Lastely, ectopic meningioma may be derived from pluripotent mesenchymal cell precursors because the meninges are mesenchymal in origin18).
Crawford et al.6), in a review of the literature, found that radiographic evidence of hyperostosis appeared in 59% of PIOMs, whereas 32% showed osteolytic changes in the surrounding bone, and 6% revealed mixed features of both osteolysis and hyperostosis. They also suggested that PIOMs are usually of the osteoblastic subtype (60%) and such this type may induce hyperostosis. The bone expansion and hyperdense skull lesions of this type of intraosseous meningioma may appear radiologically similar to en plaque meningioma, osteoma, osteosarcoma, Paget's disease, and fibrous dysplasia10). On the other hand, more rarely, PIOMs with osteolytic skull lesion occasionally showed hypodense bone feature outlined by a hypodense border zone7). The differential diagnosis of an osteolytic lesion of the skull includes fibrous dysplasia, chondroma, chondrosarcoma, epidermoid cyst, osteogenic sarcoma, myeloma, or metastatic cancer14). Also, these PIOMs with an osteolytic radiographic appearance are more likely to behave in a malignant manner (progress rapidly and invade the surrounding structures) and show anaplastic or malignant histopathology7,13).
In the study reported by Lang et al.12), preoperative imaging studies (CT or MR imaging) demonstrated dural involvement in 60% of PIOM patients. On visual inspection after craniotomy, the dura appeared normal in 40% of the cases. Changhong et al.5) observed no intraoperative dural involvement in 10 patients with PIOM. However, Bassiouni et al.3) reported that 14 out of 16 patients (88%) had a true dural involvement in PEMs of the cranial vault, of which 11 patients showed dural enhancement at the site of the tumor on preoperative MR imaging and all 14 patients demonstrated tumor infiltration of the dura on histological examination of surgical tissue samples. Therefore, they recommended that for preventing tumor recurrence that total resection of the dura at the site of craniotomy and a dural graft should be performed. In addition, dural involvement of the PIOM can be represented with the "dural tail sign" radiologically. Although dural tail sign generally was first throught to be pathognomonic of meningioma of the dural origin, it can be showed dural invasion of the tumor of intracranial or extracranial origin such as pituitary adenoma, schwannoma and astrocytoma etc8,17). Therefore, dural tail sing of our case can be explained as the results of dural involvement because it was pathologically proven as the intraosseous meningioma.
Recurrence was noted in 22% of the cases of benign PEMs found in the literature12), and PEMs located at the skull base showed a higher rate of recurrence than PEMs located along the convexity. On the other hand, a recurrence rate of 33% was reported in cases of tumors with atypical or malignant histological features12). This study presented higher mortality rates (29%) of atypical or malignant PIOM as compared to those of benign PIOM (4.8%). Out of 9 patients, 3 harbored atypical (1 case) or malignant (2 cases) tumors, 2 of which recurred (67%) and metastasized to a distant lesion; despite aggressive multimodality treatment, both patients expired. Arana et al.1) reported five recurrences (36%) within 6 months to 4 years after surgery in 14 patients in their series. Although we performed follow-up for only a short period, our case with atypical features did not show any recurrence and distant or local metastasis to the spine and the whole skull. There are another factors have been related to the prognosis except skull infiltrative types and pathological findings. Partington et al.15) first described that carcinoembrionic antigen (CEA), an oncofetal glycoprotein, was associated with atypical meningioma without secretory features. In addition, they demonstrated that the decline of CEA levels was associated with effective treatment of the symptomatic tumor.
PIOM should be distinguished from secondary involvement of the prevailing intracranial tumor and hyperostotic reactive changes. By reviewing many studies in the literature12,20,21), we suggest that PIOM generally has more tendency to form a broader base in the calvarium than that in the dura (Fig. 4A), while tumors of meningeal origin including meningioma have a broader base in the dura than in the calvarium (Fig. 4B). Accordingly, we considered that our case was of a PIOM with soft tissue and dural invasion, not of an intradural or intracranial meningioma with soft tissue and bony invasion because of the following reasons : first, the mass contents and density of the bony lesion are more than those in an extradural lesion radiologically. Second, more atypical cells were pathologically detected in the bone component than in another component. Lastly, according to the intraoperative findings, the main mass was located in the calvarial bone and the direction of mass growth was from main mass in the calvarium to dura and intradural space.
We experienced surgical treatment for a case of primary osteolytic intraosseous atypical meningioma with soft tissue and dural invasion. We believe that dural involvement in PIOM is a very important issue. These tumors generally do not infiltrate the whole layer of dura, but when they invade the whole layer of the dura as like in our case, it should be remove sufficiently infiltrated dura and bone including that underlying the normal lesion and should be frequent follow-up for prevent recurrence.
References
1. Arana E, Diaz C, Latorre FF, Menor F, Revert A, Beltrán A, et al. Primary intraosseous meningiomas. Acta Radiol. 1996; 37:937–942. PMID: 8995470.

2. Azar-Kia B, Sarwar M, Marc JA, Schechter MM. Intraosseous meningioma. Neuroradiology. 1974; 6:246–253. PMID: 4810615.

3. Bassiouni H, Asgari S, Hübschen U, König HJ, Stolke D. Dural involvement in primary extradural meningiomas of the cranial vault. J Neurosurg. 2006; 105:51–59. PMID: 16871880.

4. Borggreven PA, de Graaf FH, van der Valk P, Leemans CR. Post-traumatic cutaneous meningioma. J Laryngol Otol. 2004; 118:228–230. PMID: 15068523.

5. Changhong L, Naiyin C, Yuehuan G, Lianzhong Z. Primary intraosseous meningiomas of the skull. Clin Radiol. 1997; 52:546–549. PMID: 9240709.

6. Crawford TS, Kleinschmidt-DeMasters BK, Lillehei KO. Primary intraosseous meningioma. Case report. J Neurosurg. 1995; 83:912–915. PMID: 7472564.
7. Elder JB, Atkinson R, Zee CS, Chen TC. Primary intraosseous meningioma. Neurosurg Focus. 2007; 23:E13. PMID: 17961037.

8. Guermazi A, Lafitte F, Miaux Y, Adem C, Bonneville JF, Chiras J. The dural tail sign--beyond meningioma. Clin Radiol. 2005; 60:171–188. PMID: 15664571.

9. Hoye SJ, Hoar CS Jr, Murray JE. Extracranial meningioma presenting as a tumor of the neck. Am J Surg. 1960; 100:486–489. PMID: 13716296.

10. Jayaraj K, Martinez S, Freeman A, Lyles KW. Intraosseous meningioma-- a mimicry of Paget’s disease. J Bone Miner Res. 2001; 16:1154–1156. PMID: 11393793.
11. Kim H, Jung TY, Kim IY, Lee JK. Two cases of primary osteolytic intraosseous meningioma of the skull metastasizing to whole skull and the spine. J Korean Neurosurg Soc. 2012; 51:151–154. PMID: 22639712.

12. Lang FF, Macdonald OK, Fuller GN, DeMonte F. Primary extradural meningiomas : a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000; 93:940–950. PMID: 11117866.

13. Marwah N, Gupta S, Marwah S, Singh S, Kalra R, Arora B. Primary intraosseous meningioma. Indian J Pathol Microbiol. 2008; 51:51–52. PMID: 18417855.

14. Mattox A, Hughes B, Oleson J, Reardon D, McLendon R, Adamson C. Treatment recommendations for primary extradural meningiomas. Cancer. 2011; 117:24–38. PMID: 20824719.

15. Partington MD, Scheithauer BW, Piepgras DG. Carcinoembryonic antigen production associated with an osteolytic meningioma. Case report. J Neurosurg. 1995; 82:489–492. PMID: 7861230.

16. Politi M, Romeike BF, Papanagiotou P, Nabhan A, Struffert T, Feiden W, et al. Intraosseous hemangioma of the skull with dural tail sign : radiologic features with pathologic correlation. AJNR Am J Neuroradiol. 2005; 26:2049–2052. PMID: 16155158.
17. Rokni-Yazdi H, Sotoudeh H. Prevalence of "dural tail sign" in patients with different intracranial pathologies. Eur J Radiol. 2006; 60:42–45. PMID: 16675180.

18. Shuangshoti S. Primary meningiomas outside the central nervous system. In : Al-Mefty O, editor. Meningiomas. New York, NY: Raven Press;1991. p. 107–128.
19. Siegel GJ, Anderson PJ. Extracalvarial meningioma. Case report. J Neurosurg. 1966; 25:83–86. PMID: 5947052.
20. Tokgoz N, Oner YA, Kaymaz M, Ucar M, Yilmaz G, Tali TE. Primary intraosseous meningioma : CT and MRI appearance. AJNR Am J Neuroradiol. 2005; 26:2053–2056. PMID: 16155159.
21. Yilmaz A, Musluman M, Aydin Y. Primary osteolytic intraosseous meningioma of the frontal bone. Neurol Neurochir Pol. 2010; 44:415–418. PMID: 20827616.

Fig. 1
Initial CT and MR scans. CT scan with bone window (A) demonstrates a right-sided frontal mass expanding the calvaria with cortical destruction. Axial T2-weighted (B) MR images show the frontal intracalvarial mass lesion that was hypointense on T2-weighted images with the surrounding edema. The lesion shows intense and homogeneous enhancement on the postcontrast T1-weighted (C) image. Sagittal postcontrast T1-weighted MR images (D) reveal the intracranial extension and extradural location of the lesion.
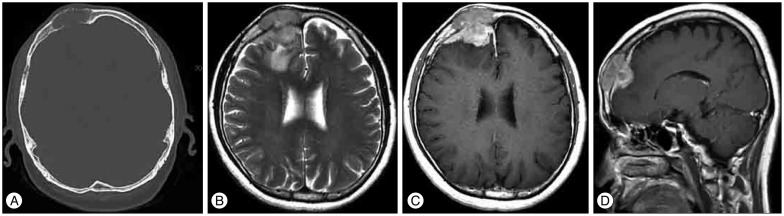
Fig. 2
Pathologic findings of the tumor specimens. Histological specimen showing atypical meningioma with freguent mitosis (A) and geographic necrosis (B) (H&E, original magnification ×100).

Fig. 3
Follow-up images 6 months after the operation. Postoperative MR image axial postcontrast T1-weighted (A) and sagittal postcontrast T1-weighted (B) MR images show no recurrence of the mass lesion except for postoperative changes.
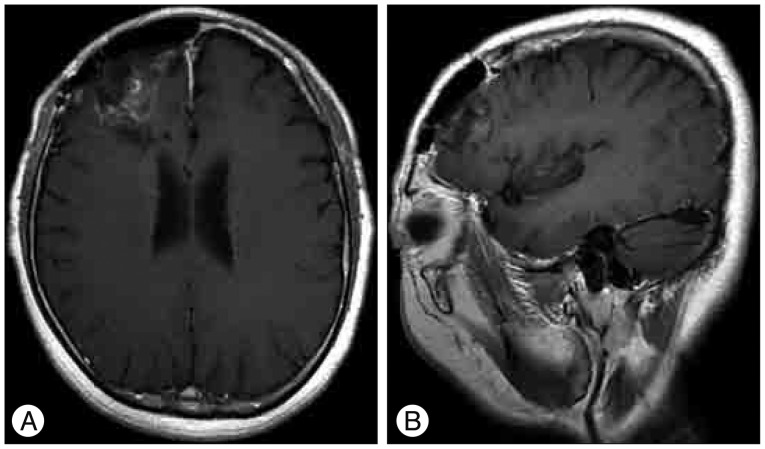
Fig. 4
Comparative illustrations of the tumor of bone origin including intraosseous meningioma (A) and the tumor of meningeal origin including intracranial meningioma (B). Prior illustration (A), which demonstrates a broad calvarial tumor base, shows more expansion of the soft tissue and dural lesion while tumor base of the next illustration (B) located in the meaninges including the dura or arachnoid and it shows more expansion of the brain, bone and a few soft tissue lesions.
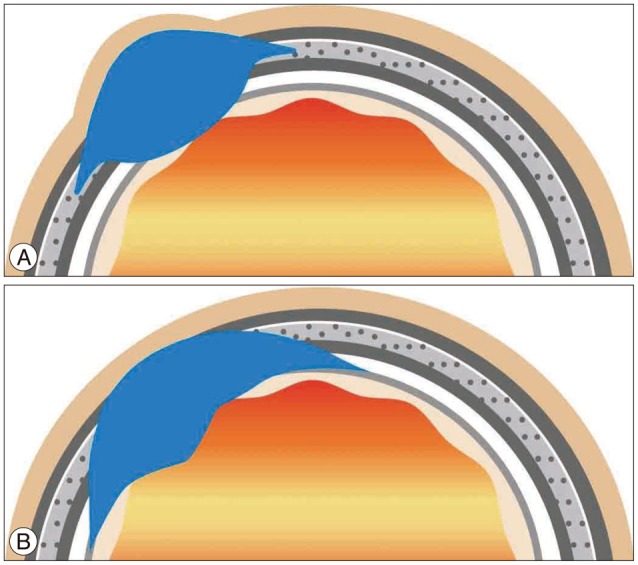
Table 1
Review of literatures for primary intraosseous osteolytic meningioma with atypical pathology and dural invasion
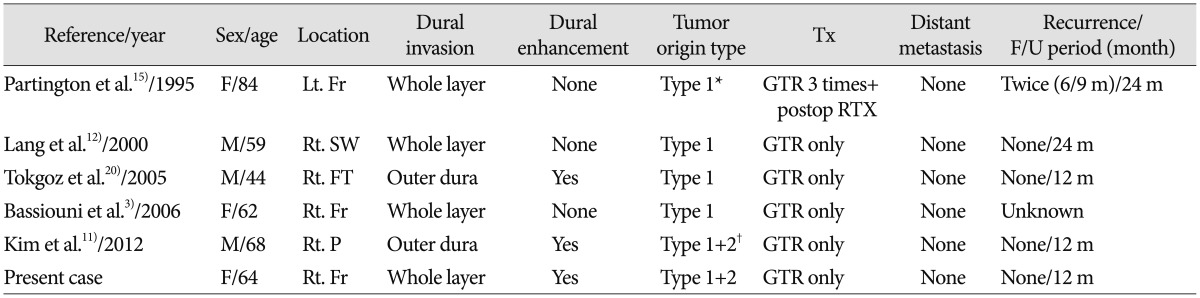
Type 1 : calvarial origin type, Type 2 : dural origin type. *First case of single origin, †First case of double origin. F : female, M : male, Lt. : left, Rt. : right, Fr : frontal, SW : sphenoid wing, FT : fronto-temporal, P : parietal, GTR : gross total resection, RTX : radiotheraphy, Tx : treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download