Abstract
Objective
The purpose of this study was to investigate the prenatal hypoxic effect on the fetal brain development.
Methods
We used the guinea pig chronic placental insufficiency model to investigate the effect of hypoxia on fetal brain development. We ligated unilateral uterine artery at 30-32 days of gestation (dg : with term defined as -67 dg). At 50 dg, 60 dg, fetuses were sacrificed and assigned to either the growth-restricted (GR) or control (no ligation) group. After fixation, dissection, and sectioning of cerebral tissue from these animals, immunohistochemistry was performed with NeuN antibody, which is a mature neuronal marker in the cerebral cortex.
Results
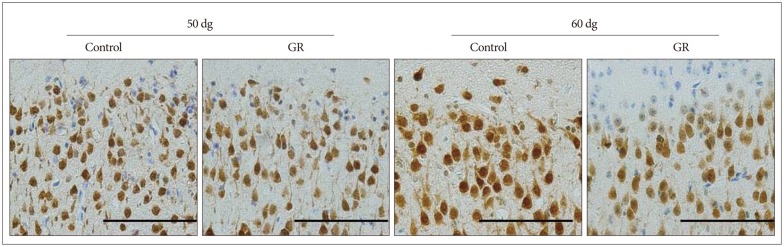
The number of NeuN-immunoreactive (IR) cells in the cerebral cortex did not differ between the GR and control groups at 50 dg. However, the number of NeuN-IR cells was lesser in GR fetuses than in controls at 60 dg (p<0.05).
Conclusion
These findings show that chronic prenatal hypoxia affect the number of neuron in the cerebral cortex of guinea pig fetus at 60 dg. The approach used in this study is helpful for extending our understanding of neurogenesis in the cerebral cortex, and the findings may be useful for elucidating the brain injury caused by prenatal hypoxia.
Chronic prenatal hypoxia results in abnormal fetal brain development which includes structural alterations. For example, total brain and cerebellum weights are reduced by 20% in the growth retarded fetus and there is an increased risk of white-matter injury5,19). These findings were resulted from the failure of the placenta to meet the increasing demands of oxygen and substrate of the developing fetus15). Very low birth weight which was affected by intrauterine growth restriction represents a major risk of life, health (hospital admissions), long-term growth, neurosensory integrity, cognitive development and learning potential10).
Neurogenesis is the process of generating new neurons from the neural stem and the progenitor cells. It is said that neurogenesis occurred in the olfactory bulb and hippocampal dentate gyrus during the embryonic period4). There were a couple of disagreements concerning the effect of hypoxia on neurogenesis. For example, cell death and apoptosis were increased at 24 hour after hypoxic injury3). Neural stem cells and oligodendrocyte progenitors in the subventricular zone (SVZ) were vulnerable to hypoxia11). On the other hands, acute hypoxic insults triggered neurogenesis in development rat brain2). After neonatal hypoxic injury, neurogenesis occurred in the SVZ9).
A previous study showed that severe acute hypoxia can promote proliferation of neuroepithelial cells in the neurogenic zone1). However it is unclear whether or not these neurons survive in cerebral cortex. We investigated the effect of hypoxia on the development of mature neuron in cerebral cortex using guinea pig chronic placental insufficiency (CPI) model.
Pregnant Dunkin-Hartley guinea pigs were provided by a certified breeder (Central Lab. Animal Inc.). Unilateral uterine artery ligation was performed as previously described at 30-32 days of gestation (dg, term approximately 67 days)13). Zoletil (10 mg/kg Virbac, S.A France) and Xylazine (0.15 mg/kg, Bayer, Germany) were injected into the muscle. After shaving the hair of the abdomen, midline incision below umbilicus was made under aseptic conditions. The ligation was performed with silk sutures (4/0). After this procedure, the abdomen was disinfected by povidine-iodine solution.
The ligation was remained in the abdomen until 50 dg or 60 dg. Fetuses at these ages were sacrificed and classified by growth-restricted (GR) and control, which was designed from the unoperated horn groups. Fetuses were removed from uterine horn and perfumed in 4% paraformaldehyde (PFA) solution. Fetal forebrains were fixed in 4% PFA at 4℃. After 2 days, the brain was embedded in paraffin. Series of 12 µm thick saggital sections were cut and mounted on gelatin coated slides (Fisher Scientific, USA).
The paraffin was removed from gelatin coated slides. The sections were washed in 0.1 M phosphate buffered saline (pH 7.4). We put slides in jars filled with 0.01 M sodium citrate buffer (pH 6.0). The slides were heated for 10 minutes and cooled down it for 10 minutes. Endogenous peroxidase was blocked by 0.3% hydrogen peroxide. Non-specific immunoreactivity was blocked with normal horse serum. Mouse anti-NeuN (1 : 100; Millipore, USA) was used as a primary antibody. A biotinylated anti-mouse IgG, the avidin-biotin-peroxidase (ABC) detection system (Vectastain ABC Elite Kit, Vector Laboratories) was used to visualize the immunoreactivity. After immunohistochemistry, the slides were counterstained by thionin and dehydrated, mounted by PolyMount medium (Polysciences, USA).
The slide was projected with a light microscope (Olympus BX41) attached digital charged couple device camera. The NeuN-immunoreactive (IR) cells were counted in the cerebral cortex including parietal cortex. Three 300-µm sections of forebrain were used for measurement of cerebral cortex. Each section was divided randomly into five areas. The number of immunoreactivity cells per defined square (mm2) was calculated in each area.
All data were analyzed using the Statistical Package for Social Sciences (Information Analysis Systems, SPSS Inc., Chicago, IL, USA). All measurements were compared between the control and GR groups using Student's t-test. The level of statistical significance was set at p<0.05.
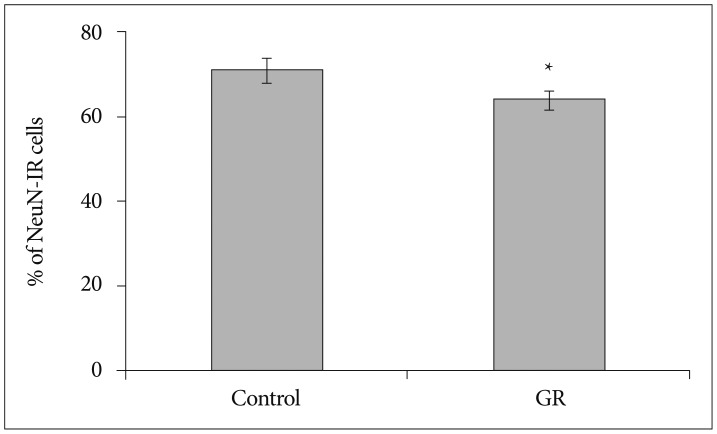
The density of NeuN-IR cells in the cerebral cortex did not differ significantly between control and GR fetuses at 50 dg (Fig. 1). The proportion of NeuN-IR cells at 50 dg did not also show significant difference (Fig. 2). However, the density and proportion of NeuN-IR cells were less in GR fetuses than in control fetuses at 60 dg (p<0.05) (Fig. 3, 4, 5).
Chronic prenatal hypoxia is considered to cause perinatal brain injury. It can result in neurological disorder such as cerebral palsy16). However, the precise prenatal hypoxic effect on the brain is still not well-known. Rees et al.17) reported that pyramidal neurons decreased in the fetal hippocampus. Mallard et al.13) also reported that number of neurons in the cerebellum reduced following intrauterine growth-restriction13). Considering these studies, chronic hypoxia had a comprehensive effect on cerebral cortex, hippocampus and cerebellum. It is known that regional distinction is important factor for determining the hypoxia effect on the brain. The present investigation has focused on the prenatal hypoxic effect on the brain. We performed uterine artery ligation to induce hypoxic status. In other study using similar way with our study, placenta blood flow was reduced by 25% at 45 dg and by 50% at 50 dg in the ligated horn7). Therefore, we expected that any changes occurred in the guinea pig fetal brain at 50 dg. However, NeuN-IR cells in the cerebrum cortex did not show any difference between control and GR group. NeuN is a mature neuron marker6,14). We performed immunohistochemistry using NeuroD, which is an early neuronal marker, at 50 dg. We rarely observed the NeuroD-IR cells in the cerebral cortex (data not shown). There was no difference between control and GR at 50 dg but the number of NeuN-IR cells was decreased in GR fetus compared to controls at 60 dg. In our previous study, we found out similar results in cerebellum at 60 dg20). The number of NeuN-IR cells reduced significantly in the development of chick brain18). In the immature rat brain, hypoxia insults induced oxidative damage to cerebral cortex21). However, there were different results in another study. For instance, there was an increase in the neurogenesis after transient hypoxia insult in the brain of the rat12). Following exposure to hypoxia, cultured neurons increased by 1 hour after reoxygenation21). Regarding the point that we used CPI model, the duration of hypoxic status was the factor of how important was the effect of hypoxia in the brain. In our study, it was shown that PCNA (cell proliferation marker)-IR cells was found in the subgranular zone of the dentate gyrus. These data suggest that survival of neurons in cerebral cortex is most likely decreased by chronic prenatal hypoxia. In adult rat, there was an induction of neurogenesis after the hypoxic damage in the forebrain8). Therefore, we plan to conduct more experiments with different ages of animals in the near future.
References
1. Cho CM, Ha SU, Bae HR, Huh JT. Endothelial cell products as a key player in hypoxia-induced nerve cell injury after stroke. J Korean Neurosurg Soc. 2006; 40:103–109.
2. Daval JL, Vert P. Apoptosis and neurogenesis after transient hypoxia in the developing rat brain. Semin Perinatol. 2004; 28:257–263. PMID: 15565785.

3. Derrick M, He J, Brady E, Tan S. The in vitro fate of rabbit fetal brain cells after acute in vivo hypoxia. J Neurosci. 2001; 21:RC138. PMID: 11264330.
4. Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, et al. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006; 199:77–91. PMID: 15916762.

5. Gross SJ, Kosmetatos N, Grimes CT, Williams ML. Newborn head size and neurological status. Predictors of growth and development of low birth weight infants. Am J Dis Child. 1978; 132:753–756. PMID: 567424.
6. Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005; 25:2518–2521. PMID: 15758160.

7. Jansson T, Thordstein M, Kjellmer I. Placental blood flow and fetal weight following uterine artery ligation. Temporal aspects of intrauterine growth retardation in the guinea pig. Biol Neonate. 1986; 49:172–180. PMID: 3955109.

8. Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001; 136:313–320. PMID: 11243473.

9. Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010; 37:267–274. PMID: 19909815.

10. Lefebvre F, Bard H, Veilleux A, Martel C. Outcome at school age of children with birthweights of 1000 grams or less. Dev Med Child Neurol. 1988; 30:170–180. PMID: 2454860.

11. Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001; 23:234–247. PMID: 11598326.

12. Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998; 18:7768–7778. PMID: 9742147.

13. Mallard C, Loeliger M, Copolov D, Rees S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience. 2000; 100:327–333. PMID: 11008170.

14. Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992; 116:201–211. PMID: 1483388.

15. Neerhof MG, Thaete LG. The fetal response to chronic placental insufficiency. Semin Perinatol. 2008; 32:201–205. PMID: 18482622.

16. Rees S, Breen S, Loeliger M, McCrabb G, Harding R. Hypoxemia near mid-gestation has long-term effects on fetal brain development. J Neuropathol Exp Neurol. 1999; 58:932–945. PMID: 10499436.

17. Rees S, Stringer M, Just Y, Hooper SB, Harding R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Brain Res Dev Brain Res. 1997; 103:103–118.

18. Rodricks CL, Gibbs ME, Castillo-Melendez M, Miller SL. The effect of hypoxia on the functional and structural development of the chick brain. Int J Dev Neurosci. 2010; 28:343–350. PMID: 20171268.

19. Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007; 130(Pt 3):654–666. PMID: 17347255.

20. So K, Chung Y, Lee H, Kim E, Jeon Y. The effect of chronic prenatal hypoxia on the development of mature neurons in the cerebellum. J Neurodev Disord. 2013; 5:17. PMID: 23822215.

21. Weis SN, Schunck RV, Pettenuzzo LF, Krolow R, Matté C, Manfredini V, et al. Early biochemical effects after unilateral hypoxia-ischemia in the immature rat brain. Int J Dev Neurosci. 2011; 29:115–120. PMID: 21255637.

Fig. 1
The density of NeuN-IR cells in the cerebral cortex from controls (n=7) and GR fetuses (n=7) at 50 dg (p>0.05). Values are expressed as a mean±SEM. SEM : standard error of mean, IR : immunoreactive, GR : growth-restricted.
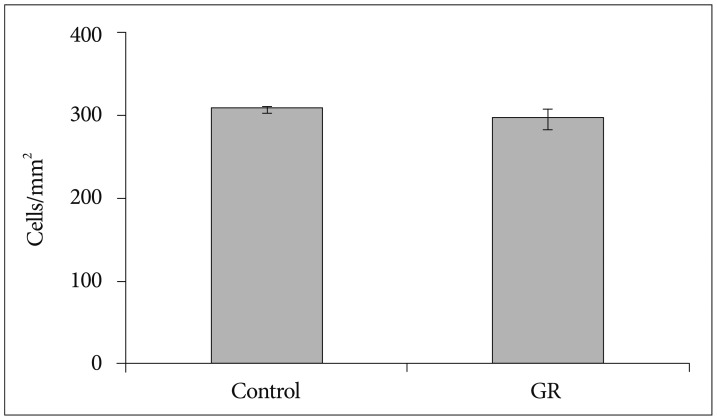
Fig. 2
The proportion of NeuN-IR cells in the cerebral cortex from controls (n=7) and GR fetuses (n=7) at 50 dg. The proportion did not differ between both groups (p>0.05). Values are expressed as a mean±SEM. SEM : standard error of mean, IR : immunoreactive, GR : growth-restricted.
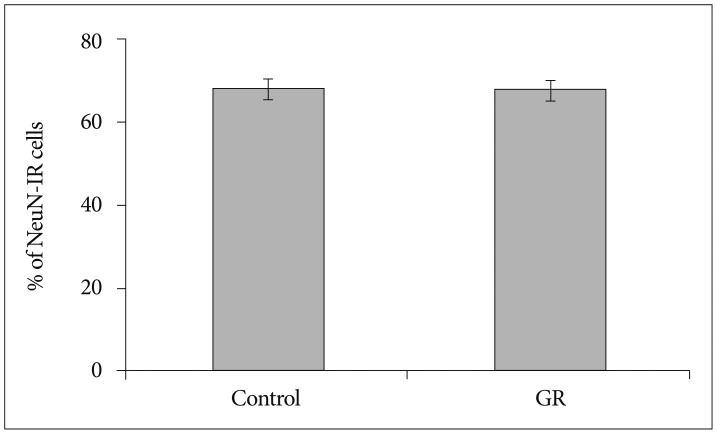
Fig. 3
The density of NeuN-IR cells in the cerebral cortex from controls and GR fetuses at 60 dg. The density of NeuN-IR cells was decreased in GR fetuses compared to controls at 60 dg (*p<0.05). Values are expressed as a mean±SEM. SEM : standard error of mean, IR : immunoreactive, GR : growth-restricted.
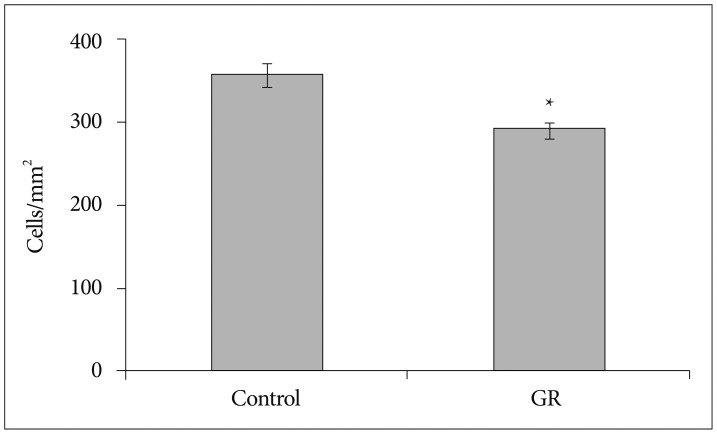
Fig. 4
The proportion of NeuN-IR cells in the cerebral cortex from controls and GR fetuses at 60 dg. The proportion of NeuN-IR cells significantly differed between control and GR group (*p<0.05). Values are expressed as a mean±SEM. SEM : standard error of mean, IR : immunoreactive, GR : growth-restricted.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download